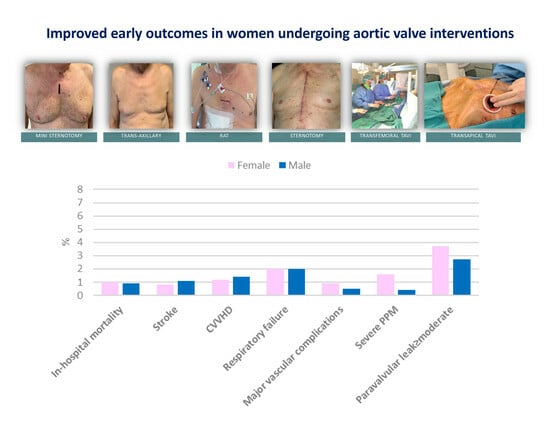
Improved Early Outcomes in Women Undergoing Aortic Valve Interventions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Population
2.3. Definitions
2.4. Surgical Aortic Valve Replacement
2.5. Trans-Catheter Aortic Valve Implantation
2.6. Echocardiographic Assessment
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Operative Data
3.3. Postoperative Outcomes
3.4. Hemodynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Pellikka, P.A. Aortic stenosis in women. Heart 2020, 106, 970–976. [Google Scholar] [CrossRef]
- Nau, D.P.; Ellis, J.J.; Kline-Rogers, E.M.; Mallya, U.; Eagle, K.A.; Erickson, S.R. Gender and perceived severity of cardiac disease: Evidence that women are “tougher”. Am. J. Med. 2005, 118, 1256–1261. [Google Scholar] [CrossRef]
- Tribouilloy, C.; Bohbot, Y.; Rusinaru, D.; Belkhir, K.; Diouf, M.; Altes, A.; Delpierre, Q.; Serbout, S.; Kubala, M.; Levy, F.; et al. Excess Mortality and Undertreatment of Women with Severe Aortic Stenosis. J. Am. Heart Assoc. 2021, 10, e018816. [Google Scholar] [CrossRef]
- Chaker, Z.; Badhwar, V.; Alqahtani, F.; Aljohani, S.; Zack, C.J.; Holmes, D.R.; Rihal, C.S.; Alkhouli, M. Sex Differences in the Utilization and Outcomes of Surgical Aortic Valve Replacement for Severe Aortic Stenosis. J. Am. Heart Assoc. 2017, 6, e006370. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- UK TAVI Trial Investigators; Toff, W.D.; Hildick-Smith, D.; Kovac, J.; Mullen, M.J.; Wendler, O.; Mansouri, A.; Rombach, I.; Abrams, K.R.; Conroy, S.P.; et al. Effect of Transcatheter Aortic Valve Implantation vs. Surgical Aortic Valve Replacement on All-Cause Mortality in Patients with Aortic Stenosis: A Randomized Clinical Trial. JAMA 2022, 327, 1875–1887. [Google Scholar]
- Skelding, K.A.; Yakubov, S.J.; Kleiman, N.S.; Reardon, M.J.; Adams, D.H.; Huang, J.; Forrest, J.K.; Popma, J.J. Transcatheter Aortic Valve Replacement Versus Surgery in Women at High Risk for Surgical Aortic Valve Replacement (from the CoreValve US High Risk Pivotal Trial). Am. J. Cardiol. 2016, 118, 560–566. [Google Scholar] [CrossRef]
- Denegri, A.; Romano, M.; Petronio, A.S.; Angelillis, M.; Giannini, C.; Fiorina, C.; Branca, L.; Barbanti, M.; Costa, G.; Brambilla, N.; et al. Gender Differences after Transcatheter Aortic Valve Replacement (TAVR): Insights from the Italian Clinical Service Project. J. Cardiovasc. Dev. Dis. 2021, 8, 114. [Google Scholar] [CrossRef]
- Malvindi, P.G.; Berretta, P.; Capestro, F.; Bifulco, O.; Alfonsi, J.; Cefarelli, M.; Pierri, M.D.; Di Eusanio, M. Results and insights after 413 TAVI procedures performed by cardiac surgeons on their own. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad074. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Hahn, R.T.; Leipsic, J.; Douglas, P.S.; Jaber, W.A.; Weissman, N.J.; Pibarot, P.; Blanke, P.; Oh, J.K. Comprehensive Echocardiographic Assessment of Normal Transcatheter Valve Function. JACC Cardiovasc. Imaging 2019, 12, 25–34. [Google Scholar] [CrossRef]
- Mayr, B.; Burri, M.; Vitanova, K.; Prinzing, A.; Goppel, G.; Krane, M.; Lange, R.; Günzinger, R. Serial echocardiographic evaluation of the Perimount Magna Ease prosthesis. J. Thorac. Dis. 2021, 13, 4104–4113. [Google Scholar] [CrossRef]
- Barnhart, G.R.; Accola, K.D.; Grossi, E.A.; Woo, Y.J.; Mumtaz, M.A.; Sabik, J.F.; Slachman, F.N.; Patel, H.J.; Borger, M.A.; Garrett, H.E., Jr.; et al. TRANSFORM (Multicenter Experience with Rapid Deployment Edwards INTUITY Valve System for Aortic Valve Replacement) US clinical trial: Performance of a rapid deployment aortic valve. J. Thorac. Cardiovasc. Surg. 2017, 153, 241–251. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, H.Y.; Sohn, S.H.; Choi, J.W.; Park, J.B.; Kim, K.H.; Kim, K.B. Hemodynamic Performance of Pericardial Bioprostheses in the Aortic Position. Korean J. Thorac. Cardiovasc. Surg. 2020, 53, 285–290. [Google Scholar] [CrossRef]
- Wyss, T.R.; Bigler, M.; Stalder, M.; Englberger, L.; Aymard, T.; Kadner, A.; Carrel, T.P. Absence of prosthesis-patient mismatch with the new generation of Edwards stented aortic bioprosthesis. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 884–887. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100029b.pdf (accessed on 9 August 2023).
- Rahimtoola, S.H. The problem of valve prosthesis-patient mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef]
- Available online: https://www.minicardiacsurgery-univpm-research.com/video-gallery (accessed on 9 August 2023).
- Berretta, P.; De Angelis, V.; Alfonsi, J.; Pierri, M.D.; Malvindi, P.G.; Zahedi, H.M.; Munch, C.; Di Eusanio, M. Enhanced recovery after minimally invasive heart valve surgery: Early and midterm outcomes. Int. J. Cardiol. 2023, 370, 98–104. [Google Scholar] [CrossRef]
- Berretta, P.; Cefarelli, M.; Montecchiani, L.; Alfonsi, J.; Vessella, W.; Zahedi, M.H.; Carozza, R.; Munch, C.; Di Eusanio, M. Minimally invasive versus standard extracorporeal circulation system in minimally invasive aortic valve surgery: A propensity score-matched study. Eur. J. Cardiothorac. Surg. 2020, 57, 717–723. [Google Scholar] [CrossRef]
- Caponcello, M.G.; Banderas, L.M.; Ferrero, C.; Bramlage, C.; Thoenes, M.; Bramlage, P. Gender differences in aortic valve replacement: Is surgical aortic valve replacement riskier and transcatheter aortic valve replacement safer in women than in men? J. Thorac. Dis. 2020, 12, 3737–3746. [Google Scholar] [CrossRef]
- Elhmidi, Y.; Piazza, N.; Mazzitelli, D.; Wottke, M.; Lange, R.; Bleiziffer, S. Sex-related differences in 2197 patients undergoing isolated surgical aortic valve replacement. J. Card. Surg. 2014, 29, 772–778. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Dangas, G.; Yu, J.; Vemulapalli, S.; Suchindran, S.; Vora, A.N.; Baber, U.; Mehran, R.; STS/ACC TVT Registry. Sex-Based Differences in Outcomes with Transcatheter Aortic Valve Therapy: TVT Registry from 2011 to 2014. J. Am. Coll. Cardiol. 2016, 68, 2733–2744. [Google Scholar] [CrossRef]
- Humphries, K.H.; Toggweiler, S.; Rodés-Cabau, J.; Nombela-Franco, L.; Dumont, E.; Wood, D.A.; Willson, A.B.; Binder, R.K.; Freeman, M.; Lee, M.K.; et al. Sex differences in mortality after transcatheter aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2012, 60, 882–886. [Google Scholar] [CrossRef]
- O’Connor, S.A.; Morice, M.C.; Gilard, M.; Leon, M.B.; Webb, J.G.; Dvir, D.; Rodés-Cabau, J.; Tamburino, C.; Capodanno, D.; D’Ascenzo, F.; et al. Revisiting Sex Equality with Transcatheter Aortic Valve Replacement Outcomes: A Collaborative, Patient-Level Meta-Analysis of 11,310 Patients. J. Am. Coll. Cardiol. 2015, 66, 221–228. [Google Scholar] [CrossRef]
- Baran, J.; Podolec, J.; Tomala, M.T.; Nawrotek, B.; Niewiara, Ł.; Gackowski, A.; Przewłocki, T.; Żmudka, K.; Kabłak-Ziembicka, A. Increased risk profile in the treatment of patients with symptomatic degenerative aortic valve stenosis over the last 10 years. Postep. Kardiol Interwencyjnej 2018, 14, 276–284. [Google Scholar] [CrossRef]
- Kapetanakis, E.I.; Athanasiou, T.; Mestres, C.A.; Nashef, S.A.; Aagaard, J.; Moritz, A.; Van Ingen, G.; Chronidou, F.; Palatianos, G.; Alivizatos, P.A.; et al. Aortic valve replacement: Is there an implant size variation across Europe? J. Heart Valve Dis. 2008, 17, 200–205. [Google Scholar]
- Franzen, S.F.; Huljebrant, I.E.; Konstantinov, I.E.; Nylander, E.; Olin, C.L. Aortic valve replacement for aortic stenosis in patients with small aortic root. J. Heart Valve Dis. 1996, 5 (Suppl. 3), S284–S288. [Google Scholar]
- Panoulas, V.F.; Chandrasekhar, J.; Busi, G.; Ruparelia, N.; Zhang, Z.; Mehilli, J.; Sartori, S.; Lefèvre, T.; Presbitero, P.; Capranzano, P.; et al. Prevalence, predictors, and outcomes of patient prosthesis mismatch in women undergoing TAVI for severe aortic stenosis: Insights from the WIN-TAVI registry. Catheter Cardiovasc. Interv. 2021, 97, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.B.O.; de Carvalho, M.M.B.; Sobral Filho, D.C.; Cavalcanti, L.R.P.; Rayol, S.D.C.; Diniz, R.G.S.; Menezes, A.M.; Clavel, M.A.; Pibarot, P.; Lima, R.C. Surgical aortic valve replacement and patient-prosthesis mismatch: A meta-analysis of 108 182 patients. Eur. J. Cardiothorac. Surg. 2019, 56, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.; Malvindi, P.G.; Olevano, C.; Zingale, A.; Salem, H.; Ohri, S.K. Impact of valve size, predicted effective and indexed effective orifice area after aortic valve replacement. J. Card. Surg. 2021, 36, 961–968. [Google Scholar] [CrossRef]
- Schofer, N.; Deuschl, F.; Rübsamen, N.; Skibowski, J.; Seiffert, M.; Voigtländer, L.; Schaefer, A.; Schneeberger, Y.; Schirmer, J.; Reichenspurner, H.; et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation: Prevalence and prognostic impact with respect to baseline left ventricular function. EuroIntervention 2019, 14, 1648–1655. [Google Scholar] [CrossRef]
- Pibarot, P.; Weissman, N.J.; Stewart, W.J.; Hahn, R.T.; Lindman, B.R.; McAndrew, T.; Kodali, S.K.; Mack, M.J.; Thourani, V.H.; Miller, D.C.; et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: A PARTNER trial cohort—A analysis. J. Am. Coll. Cardiol. 2014, 64, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Umemoto, T.; ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Prosthesis-Patient Mismatch After Transcatheter Aortic Valve Implantation. Ann. Thorac. Surg. 2016, 101, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Rodés-Cabau, J.; Dumont, É.; Bagur, R.; Bergeron, S.; De Larochellière, R.; Doyle, D.; Larose, E.; Dumesnil, J.G.; Pibarot, P. Validation and characterization of transcatheter aortic valve effective orifice area measured by Doppler echocardiography. JACC Cardiovasc. Imaging 2011, 4, 1053–1062. [Google Scholar] [CrossRef]
- Deharo, P.; Leroux, L.; Theron, A.; Ferrara, J.; Vaillier, A.; Jaussaud, N.; Porto, A.; Morera, P.; Gariboldi, V.; Iung, B.; et al. Long-Term Prognosis Value of Paravalvular Leak and Patient-Prosthesis Mismatch following Transcatheter Aortic Valve Implantation: Insight from the France-TAVI Registry. J. Clin. Med. 2022, 11, 6117. [Google Scholar] [CrossRef]
- Kolkailah, A.A.; Hirji, S.A.; Ejiofor, J.I.; Del Val, F.R.; Chowdhury, R.; McGurk, S.; Lee, J.; Kaneko, T. Impact of Prosthesis Size and Prosthesis-Patient Mismatch on Outcomes in Younger Female Patients Undergoing Aortic Valve Replacement. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 219–228. [Google Scholar] [CrossRef]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef]
- Isaacs, A.J.; Shuhaiber, J.; Salemi, A.; Isom, O.W.; Sedrakyan, A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J. Thorac. Cardiovasc. Surg. 2015, 149, 1262–1269. [Google Scholar] [CrossRef]
- Malvindi, P.G.; Luthra, S.; Olevano, C.; Salem, H.; Kowalewski, M.; Ohri, S. Aortic valve replacement with biological prosthesis in patients aged 50-69 years. Eur. J. Cardiothorac. Surg. 2021, 59, 1077–1086. [Google Scholar] [CrossRef]
- Abdelghani, M.; Mankerious, N.; Allali, A.; Landt, M.; Kaur, J.; Sulimov, D.S.; Merten, C.; Sachse, S.; Mehilli, J.; Neumann, F.J.; et al. Bioprosthetic Valve Performance After Transcatheter Aortic Valve Replacement with Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights from the CHOICE Trial and the CHOICE-Extend Registry. JACC Cardiovasc. Interv. 2018, 11, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.; Theron, A.; Porto, A.; Morera, P.; Luporsi, P.; Jaussaud, N.; Gariboldi, V.; Collart, F.; Cuisset, T.; Deharo, P. Prosthesis-Patient Mismatch in Small Aortic Annuli: Self-Expandable vs. Balloon-Expandable Transcatheter Aortic Valve Replacement. J. Clin. Med. 2022, 11, 1959. [Google Scholar] [CrossRef]
- Dismorr, M.; Glaser, N.; Franco-Cereceda, A.; Sartipy, U. Effect of Prosthesis-Patient Mismatch on Long-Term Clinical Outcomes after Bioprosthetic Aortic Valve Replacement. J. Am. Coll. Cardiol. 2023, 81, 964–975. [Google Scholar] [CrossRef]
- Geisler, D.; Rudziński, P.N.; Hasan, W.; Andreas, M.; Hasimbegovic, E.; Adlbrecht, C.; Winkler, B.; Weiss, G.; Strouhal, A.; Delle-Karth, G.; et al. Identifying Patients without a Survival Benefit following Transfemoral and Transapical Transcatheter Aortic Valve Replacement. J. Clin. Med. 2021, 10, 4911. [Google Scholar] [CrossRef]
- Baran, J.; Kablak-Ziembicka, A.; Kleczynski, P.; Alfieri, O.; Niewiara, Ł.; Badacz, R.; Pieniazek, P.; Legutko, J.; Zmudka, K.; Przewlocki, T.; et al. Association of Increased Vascular Stiffness with Cardiovascular Death and Heart Failure Episodes Following Intervention on Symptomatic Degenerative Aortic Stenosis. J. Clin. Med. 2022, 11, 2078. [Google Scholar] [CrossRef]
- Vogl, B.J.; El Shaer, A.; Crestanello, J.A.; Alkhouli, M.; Hatoum, H. Flow dynamics in the sinus and downstream of third and fourth generation balloon expandable transcatheter aortic valves. J. Mech. Behav. Biomed. Mater. 2022, 127, 105092. [Google Scholar] [CrossRef]
- Ternacle, J.; Guimaraes, L.; Vincent, F.; Côté, N.; Côté, M.; Lachance, D.; Clavel, M.A.; Abbas, A.E.; Pibarot, P.; Rodés-Cabau, J. Reclassification of prosthesis-patient mismatch after transcatheter aortic valve replacement using predicted vs. measured indexed effective orifice area. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 11–20. [Google Scholar] [CrossRef]
- Jørgensen, T.H.; Thyregod, H.G.H.; Ihlemann, N.; Nissen, H.; Petursson, P.; Kjeldsen, B.J.; Steinbrüchel, D.A.; Olsen, P.S.; Søndergaard, L. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur. Heart J. 2021, 42, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Hildick-Smith, D.; Parker, J.; Malkin, C.J.; Cunnington, M.S.; Gurung, S.; Mailey, J.; MacCarthy, P.A.; Bharucha, A.; Brecker, S.J.; et al. Long-term durability of self-expanding and balloon-expandable transcatheter aortic valve prostheses: UK TAVI registry. Catheter. Cardiovasc. Interv. 2023, 101, 932–942. [Google Scholar] [CrossRef] [PubMed]


| Variable | Female (n = 514) N (%) or Median (IQR 1–3) | Male (n = 441) N (%) or Median (IQR 1–3) | p Value |
|---|---|---|---|
| Age (years) | 80 (75–84) | 77 (71–83) | <0.001 |
| BMI (kg/m2) | 26 (23.4–29.7) | 27 (24.5–29.4) | 0.015 |
| BSA (m2) | 1.7 (1.6–1.8) | 1.9 (1.8–2.0) | <0.001 |
| Euroscore II (%) | 2 (1.4–3.4) | 1.6 (1.0–2.6) | <0.001 |
| NYHA class III–IV | 279 (55) | 218 (49) | 0.14 |
| Hypertension | 427 (84) | 368 (84) | 0.80 |
| Diabetes | 121 (24) | 114 (26) | 0.49 |
| Dyslipidemia | 283 (56) | 249 (56) | 0.88 |
| COPD | 80 (16) | 89 (20) | 0.06 |
| Atrial fibrillation | 100 (19) | 89 (20) | 0.77 |
| Pacemaker | 14 (3) | 29 (7) | 0.046 |
| eGFR < 50 | 231 (45) | 165 (37) | 0.018 |
| Dialysis | 4 (1) | 9 (2) | 0.10 |
| History of CAD | 122 (24) | 172 (39) | <0.001 |
| Previous AMI | 22 (4) | 48 (11) | <0.001 |
| Previous PCI | 54 (11) | 98 (22) | <0.001 |
| Previous CABG | 10 (4) | 33 (7) | 0.034 |
| Previous CVA | 64 (13) | 54 (12) | 0.92 |
| Peripheral arteriopathy | 49 (10) | 62 (14) | 0.029 |
| TAVI | SAVR | |||||
|---|---|---|---|---|---|---|
| Variable | Female (n = 276) N (%) or Median (IQR 1–3) | Male (n = 204) N (%) or Median (IQR 1–3) | p Value | Female (n = 238) N (%) or Median (IQR 1–3) | Male (n = 237) N (%) or Median (IQR 1–3) | p Value |
| Age (years) | 83 (80–86) | 82 (79–85) | 0.20 | 75 (70–79) | 72 (67–76) | <0.001 |
| BMI (kg/m2) | 25.3 (22.5–28.7) | 26.0 (24.1–29.1) | 0.009 | 27.1 (24.3–30.5) | 27.3 (24.8–29.6) | 0.63 |
| BSA (m2) | 1.7 (1.6–1.8) | 1.9 (1.8–2.0) | <0.001 | 1.7 (1.6–1.8) | 1.9 (1.8–2.0) | <0.001 |
| Euroscore II (%) | 2.8 (1.8–4.1) | 2.5 (1.6–4.7) | 0.72 | 1.5 (1.2–2.4) | 1.1 (0.8–1.6) | <0.001 |
| NYHA class III–IV | 167 (61) | 129 (63) | 0.54 | 112 (47) | 89 (38) | 0.04 |
| Hypertension | 226 (83) | 166 (81) | 0.51 | 201 (85) | 202 (85) | 0.90 |
| Diabetes | 66 (24) | 61 (30) | 0.19 | 55 (23) | 53 (22) | 0.90 |
| Dyslipidemia | 142 (52) | 129 (63) | 0.029 | 141 (59) | 120 (51) | 0.06 |
| COPD | 59 (22) | 50 (25) | 0.41 | 21 (9) | 39 (17) | 0.001 |
| Atrial fibrillation | 65 (24) | 64 (32) | 0.05 | 35 (15) | 25 (11) | 0.21 |
| Pacemaker | 11 (4) | 22 (11) | 0.003 | 3 (1.3) | 7 (3) | 0.22 |
| eGFR < 50 | 161 (58) | 115 (56) | 0.67 | 70 (29) | 50 (21) | 0.037 |
| Dialysis | 4 (1.5) | 6 (3) | 0.33 | 0 | 3 (1) | 0.12 |
| History of CAD | 80 (29) | 114 (56) | <0.001 | 42 (18) | 58 (25) | 0.07 |
| Previous AMI | 12 (4) | 30 (15) | <0.001 | 10 (4) | 18 (8) | 0.17 |
| Previous PCI | 45 (16) | 68 (33) | <0.001 | 9 (4) | 30 (13) | <0.001 |
| Previous CABG | 10 (4) | 33 (16) | <0.001 | - | - | - |
| Previous CVA | 46 (17) | 30 (15) | 0.56 | 18 (8) | 24 (10) | 0.34 |
| Peripheral arteriopathy | 35 (14) | 44 (22) | 0.009 | 14 (6) | 18 (8) | 0.47 |
| Variable | Female (n = 276) | Male (n = 204) | p Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Type of prostheses | 0.07 | ||||
| 169 | 61 | 141 | 69 | |
| 107 | 39 | 63 | 31 | |
| Size of prosthesis | <0.001 | ||||
| 9 | 3 | 0 | 0 | |
| 119 | 43 | 20 | 10 | |
| 99 | 36 | 86 | 42 | |
| 47 | 17 | 69 | 34 | |
| 2 | 1 | 29 | 14 | |
| Access | 0.043 | ||||
| Transapical access | 40 | 14 | 44 | 22 | |
| Transfemoral access | 236 | 86 | 160 | 78 | |
| Variable | Female (n = 238) | Male (n = 237) | p Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Type of prosthesis | <0.001 | ||||
| 125 | 53 | 181 | 89 | |
| 53 | 22 | 18 | 9 | |
| 60 | 25 | 5 | 2 | |
| Size of prosthesis | <0.001 | ||||
| 54 | 23 | 3 | 1 | |
| 128 | 54 | 21 | 9 | |
| 50 | 21 | 110 | 46 | |
| 6 | 3 | 79 | 34 | |
| 0 | 0 | 17 | 7 | |
| 0 | 0 | 7 | 3 | |
| Minimally invasive approach | 196 | 82 | 192 | 81 | 0.71 |
| Full sternotomy | 42 | 18 | 45 | 19 | |
| Cross-clamp time | 53 (43–64) | 57 (47–67) | 0.017 | ||
| CBP time | 70 (60–82) | 75 (62–88) | 0.036 | ||
| Variable | Female (n = 514) N (%) or Median (IQR 1–3) | Male (n = 441) N (%) or Median (IQR 1–3) | p Value |
|---|---|---|---|
| In-hospital mortality | 5 (1) | 4 (0.9) | 0.81 |
| Stroke | 4 (0.8) | 5 (1.1) | 0.57 |
| Renal failure | 38 (7.5) | 48 (11) | 0.06 |
| CVVH | 6 (1.2) | 6 (1.4) | 0.79 |
| AMI | 1 (0.2) | 1 (0.2) | 0.91 |
| Respiratory insufficiency | 10 (2) | 8 (2) | 0.88 |
| Atrial fibrillation (in patients with preop SR) | 97/414 (23) | 95/352 (27) | 0.46 |
| Definitive pacemaker | 52/500 (10) | 68 (16.5) | 0.006 |
| Vascular complications | 0.18 | ||
| Major | 5 (0.9) | 2 (0.5) | |
| Minor | 20 (3.9) | 12 (2.7) | |
| Intubation time (hours) | 5 (0–8) | 5 (0–8) | 0.28 |
| ICU stay (hours) | 24 (6–29) | 24 (17–46) | 0.008 |
| Hospital stay (days) | 6 (5–7) | 6 (5–8) | 0.24 |
| TAVI | SAVR | |||||
|---|---|---|---|---|---|---|
| Variable | Female (n = 276) N (%) or Median (IQR 1–3) | Male (n = 204) N (%) or Median (IQR 1–3) | p Value | Female (n = 238) N (%) or Median (IQR 1–3) | Male (n = 237) N (%) or Median (IQR 1–3) | p Value |
| In-hospital mortality | 4 (1.4) | 3 (1.5) | 0.99 | 1 (0.4) | 1 (0.4) | 0.99 |
| Stroke | 3 (1.1) | 5 (2.5) | 0.29 | 1 (0.4) | 0 | 0.99 |
| Renal failure | 24 (9) | 29 (14) | 0.06 | 14 (6) | 19 (8) | 0.37 |
| CVVH | 3 (1) | 3 (1.5) | 0.70 | 3 (1.3) | 3 (1.3) | 0.99 |
| AMI | 0 | 0 | - | 1 (0.4) | 1 (0.4) | 0.99 |
| Respiratory insufficiency | 5 (1.8) | 4 (2) | 0.99 | 5 (2.1) | 4 (1.7) | 0.99 |
| Atrial fibrillation | 21/211 (10) | 16/139 (12) | 0.70 | 76/203 (37) | 79/213 (37) | 0.99 |
| Definitive pacemaker | 46/265 (17) | 56/182 (30) | <0.001 | 6 (3) | 12/230 (5) | 0.15 |
| Vascular complications | 0.18 | - | ||||
| Major | 5 (2) | 2 (1) | 0 | 0 | - | |
| Minor | 20 (7) | 12 (6) | 0 | 0 | - | |
| Intubation time (hours) | * | * | 5 (0–8) | 5 (0–8) | 0.63 | |
| ICU stay (hours) | ** | ** | 24 (23–48) | 24 (23–48) | 0.35 | |
| Hospital stay (days) | 6 (5–7) | 6 (5–8) | 0.13 | 6 (5–8) | 6 (5–7) | 0.37 |
| Variable | Female (n = 514) N (%) or Median (IQR 1–3) | Male (n = 441) N (%) or Median (IQR 1–3) | p Value |
|---|---|---|---|
| Preoperative peak gradient (mmHg) | 80 (70–99) | 76 (67–90) | <0.001 |
| Preoperative mean gradient (mmHg) | 50 (41–60) | 46 (41–55) | <0.001 |
| AVA index (cm2) | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) | 0.28 |
| Preoperative LVEF (%) | 60 (55–65) | 60 (50–63) | <0.001 |
| Postoperative peak gradient (mmHg) | 21 (16–27) | 20 (16–25) | 0.019 |
| Postoperative mean gradient (mmHg) | 11 (9–15) | 10 (8–13) | 0.007 |
| Postoperative LVEF (%) | 60 (55–65) | 57 (50–60) | 0.024 |
| Paravalvular leak | 0.40 | ||
| Moderate | 16 (3.1) | 12 (2.7) | |
| Severe | 3 (0.6) | 0 | |
| PPM | 135 (26.3) | 43 (9.8) | <0.001 |
| Severe PPM | 8 (1.6) | 2 (0.4) | 0.18 |
| TAVI | SAVR | |||||
|---|---|---|---|---|---|---|
| Variable | Female (n = 276) N (%) or Median (IQR 1–3) | Male (n = 204) N (%) or Median (IQR 1–3) | p Value | Female (n = 238) N (%) or Median (IQR 1–3) | Male (n = 237) N (%) or Median (IQR 1–3) | p Value |
| Preoperative peak gradient (mmHg) | 78 (68–97) | 73 (64–85) | <0.001 | 84 (72–101) | 80 (70–95) | 0.05 |
| Preoperative mean gradient (mmHg) | 48 (41–60) | 45 (40–52) | 0.001 | 52 (42–63) | 49 (41–59) | 0.034 |
| AVA index (cm2) | 0.4 (0.4–0.6) | 0.4 (0.4–0.5) | 0.62 | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) | 0.28 |
| Preoperative LVEF (%) | 60 (55–65) | 56 (45–61) | <0.001 | 60 (58–65) | 60 (55–65) | 0.002 |
| Postoperative peak gradient (mmHg) | 20 (15–24) | 19 (14–23) | 0.09 | 23 (18–29) | 20 (17–26) | 0.016 |
| Postoperative mean gradient (mmHg) | 11 (8–13) | 10 (7–12) | 0.002 | 12 (10–16) | 11 (9–15) | 0.033 |
| Postoperative LVEF (%) | 60 (55–65) | 55 (48–60) | <0.001 | 60 (55–65) | 60 (55–63) | 0.024 |
| Paravalvular leak | 0.47 | 0.25 | ||||
| Moderate | 16 (5.8) | 9 (4.4) | 0 | 3 (1.3) | ||
| Severe | 3 (1.1) | 0 | 0 | 0 | ||
| PPM | 72 (26) | 31 (15) | 0.004 | 63 (27) | 12 (5) | <0.001 |
| Severe PPM | 2 (0.7) | 1 (0.5) | 0.79 | 6 (2.5) | 1 (0.4) | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvindi, P.G.; Bifulco, O.; Berretta, P.; Alfonsi, J.; Cefarelli, M.; Zingaro, C.; Capestro, F.; D’Alfonso, A.; Di Eusanio, M. Improved Early Outcomes in Women Undergoing Aortic Valve Interventions. J. Clin. Med. 2023, 12, 5749. https://doi.org/10.3390/jcm12175749
Malvindi PG, Bifulco O, Berretta P, Alfonsi J, Cefarelli M, Zingaro C, Capestro F, D’Alfonso A, Di Eusanio M. Improved Early Outcomes in Women Undergoing Aortic Valve Interventions. Journal of Clinical Medicine. 2023; 12(17):5749. https://doi.org/10.3390/jcm12175749
Chicago/Turabian StyleMalvindi, Pietro Giorgio, Olimpia Bifulco, Paolo Berretta, Jacopo Alfonsi, Mariano Cefarelli, Carlo Zingaro, Filippo Capestro, Alessandro D’Alfonso, and Marco Di Eusanio. 2023. "Improved Early Outcomes in Women Undergoing Aortic Valve Interventions" Journal of Clinical Medicine 12, no. 17: 5749. https://doi.org/10.3390/jcm12175749
APA StyleMalvindi, P. G., Bifulco, O., Berretta, P., Alfonsi, J., Cefarelli, M., Zingaro, C., Capestro, F., D’Alfonso, A., & Di Eusanio, M. (2023). Improved Early Outcomes in Women Undergoing Aortic Valve Interventions. Journal of Clinical Medicine, 12(17), 5749. https://doi.org/10.3390/jcm12175749