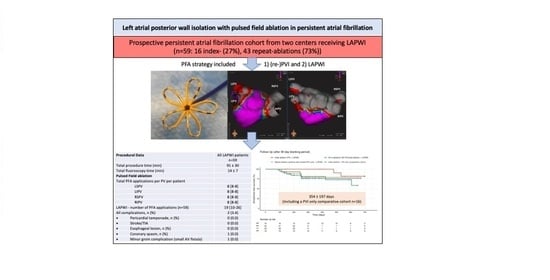
Left Atrial Posterior Wall Isolation with Pulsed Field Ablation in Persistent Atrial Fibrillation
Abstract
:1. Introduction
2. Methods
2.1. Study Design
- (1)
- The LAPWI cohort, including first-time and repeat ablation procedures in persistent AF patients. Ablation strategy included re-(PVI) with LAPWI performed by PFA.
- (2)
- The comparative PVI-only cohort, including first-time ablation procedures in persistent AF patients. Ablation strategy included a PVI-only approach without LAPWI, performed by PFA.
2.2. Catheter Ablation—1. Procedural Workflow of LAPWI Cohort
2.3. Catheter Ablation—2. Procedural Workflow of the Comparative PVI-Only Cohort
2.4. Pulsed Field Ablation
2.5. Follow-Up
2.6. Study Endpoints
2.7. Statistics
3. Results
3.1. LAPWI Cohort—Baseline Characteristics
3.2. LAPWI Cohort—PFA Ablation Procedure
3.3. LAPWI Cohort—Procedural Adverse Events
3.4. Results—Comparative PVI-Only Cohort
3.5. Follow-Up—Complete Study Cohort
4. Discussion
4.1. Major Findings
4.2. Left Atrial Posterior Wall Isolation with PFA
4.3. Thermal Energy Sources for LAPWI
4.4. Safety Regarding the Esophagus
4.5. PFA in the Setting of Re-Ablation
4.6. Durable Pulmonary Vein Isolation
4.7. Future Directions
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| CA | Catheter ablation |
| LA | Left atrium/left atrial |
| LAPWI | Left atrial posterior wall isolation |
| LIPV | Left inferior pulmonary vein |
| LSPV | Left superior pulmonary vein |
| PersAF | Persistent atrial fibrillation |
| PFA | Pulsed Field Ablation |
| PV | Pulmonary vein |
| PVI | Pulmonary vein isolation |
| Pts | Patients |
| RIPV | Right inferior pulmonary vein |
| RSPV | Right superior pulmonary vein |
References
- Hindricks, G.; Potpara, T.; Serbia, C.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Willems, S.; Borof, K.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Gessler, N.; Goette, A.; Haegeli, L.M.; et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: The EAST-AFNET 4 trial. Eur. Heart J. 2021, 27, ehab593. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, L.; Sehner, S.; Suling, A.; Borof, K.; Breithardt, G.; Crijns, H.; Goette, A.; Wegscheider, K.; Zapf, A.; Camm, J.; et al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: The EAST-AFNET 4 trial. Eur. Heart J. 2022, 43, 4127–4144. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wazni, O.; McGann, C.; Greene, T.; Dean, J.M.; Dagher, L.; Kholmovski, E.; Mansour, M.; Marchlinski, F.; Wilber, D.; et al. Effect of MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The DECAAF II Randomized Clinical Trial. JAMA 2022, 327, 2296–2305. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Douglas, Y.L.; Jongbloed, M.R.M.; Gittenberger-de Groot, A.C.; Evers, D.; Dion, R.A.E.; Voigt, P.; Bartelings, M.M.; Schalij, M.J.; Ebels, T.; DeRuiter, M.C. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am. J. Cardiol. 2006, 97, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, H.; Thomas, S.P.; Prabhu, S. How to perform posterior wall isolation in catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 345–352. [Google Scholar] [CrossRef]
- Ho, S.Y.; Cabrera, J.A.; Sanchez-Quintana, D. Left atrial anatomy revisited. Circ. Arrhythm. Electrophysiol. 2012, 5, 220–228. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kuwahara, T.; Kobori, A.; Takahashi, Y.; Takei, A.; Sato, A.; Isobe, M.; Takahashi, A. Long-term clinical outcome of extensive pulmonary vein isolation-based catheter ablation therapy in patients with paroxysmal and persistent atrial fibrillation. Heart 2011, 97, 668–673. [Google Scholar] [CrossRef]
- Romero, J.; Gabr, M.; Alviz, I.; Briceno, D.; Diaz, J.C.; Rodriguez, D.; Patel, K.; Polanco, D.; Trivedi, C.; Mohanty, S.; et al. Focal impulse and rotor modulation guided ablation versus pulmonary vein isolation for atrial fibrillation: A meta-analysis of head-to-head comparative studies. J. Cardiovasc. Electrophysiol. 2021, 32, 1822–1832. [Google Scholar] [CrossRef]
- Jalife, J. Rotors and spiral waves in atrial fibrillation. J. Cardiovasc. Electrophysiol. 2003, 14, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Kistler, P.M.; Chieng, D.; Sugumar, H.; Ling, L.-H.; Segan, L.; Azzopardi, S.; Al-Kaisey, A.; Parameswaran, R.; Anderson, R.D.; Hawson, J.; et al. Effect of Catheter Ablation Using Pulmonary Vein Isolation With vs Without Posterior Left Atrial Wall Isolation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The CAPLA Randomized Clinical Trial. JAMA 2023, 329, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Gaspar, T.; Schönbauer, R.; Wójcik, M.; Fiedler, L.; Roithinger, F.X.; Martinek, M.; Pürerfellner, H.; Kirstein, B.; Richter, U.; et al. Low-Voltage Myocardium-Guided Ablation Trial of Persistent Atrial Fibrillation. NEJM Evid. 2022, 1, EVIDoa2200141. [Google Scholar] [CrossRef]
- DeLurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ. Arrhythm. Electrophysiol. 2020, 13, e009288. [Google Scholar] [CrossRef] [PubMed]
- Zellerhoff, S.; Ullerich, H.; Lenze, F.; Meister, T.; Wasmer, K.; Mönnig, G.; Köbe, J.; Milberg, P.; Bittner, A.; Domschke, W.; et al. Damage to the esophagus after atrial fibrillation ablation: Just the tip of the iceberg? High prevalence of mediastinal changes diagnosed by endosonography. Circ. Arrhythm. Electrophysiol. 2010, 3, 155–159. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Anic, A.; Koruth, J.; Petru, J.; Funasako, M.; Minami, K.; Breskovic, T.; Sikiric, I.; Dukkipati, S.R.; Kawamura, I.; et al. Pulsed Field Ablation in Patients With Persistent Atrial Fibrillation. JACC 2020, 76, 1068–1080. [Google Scholar] [CrossRef]
- Gunawardene, M.A.; Schaeffer, B.N.; Jularic, M.; Eickholt, C.; Maurer, T.; Pape, U.F.; Hartmann, J.; Flindt, M.; Anwar, O.; Willems, S. Pulsed—Field ablation combined with ultrahigh—Density mapping in patients undergoing catheter ablation for atrial fibrillation: Practical and electrophysiological considerations. J. Cardiovasc. Electrophysiol. 2022, 33, 345–356. [Google Scholar] [CrossRef]
- Ekanem, E.; Reddy, V.Y.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Scherr, D.; Wakili, R.; et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022, 24, 1256–1266. [Google Scholar] [CrossRef]
- Jiang, X.; Liao, J.; Ling, Z.; Meyer, C.; Sommer, P.; Futyma, P.; Martinek, M.; Schratter, A.; Acou, W.-J.; Wang, J.; et al. Adjunctive Left Atrial Posterior Wall Isolation in Treating Atrial Fibrillation: Insight From a Large Secondary Analysis. JACC Clin. Electrophysiol. 2022, 8, 605–618. [Google Scholar] [CrossRef]
- Calvert, P.; Lip, G.Y.H.; Gupta, D. Radiofrequency catheter ablation of atrial fibrillation: A review of techniques. Trends Cardiovasc. Med. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Worck, R.; Sørensen, S.K.; Johannessen, A.; Ruwald, M.; Haugdal, M.; Hansen, J. Posterior wall isolation in persistent atrial fibrillation feasibility, safety, durability, and efficacy. J. Cardiovasc. Electrophysiol. 2022, 33, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Schricker, A.A.; Nerlekar, R.; Feng, Z.; Sudat, S.; Cook, K.; Salcedo, J.; Engel, G.; Winkle, R.A.; Woods, C. The Posterior Wall Isolation for Persistent Atrial Fibrillation High-Power Short Duration (PEF-HOT) TRIAL. JACC Clin. Electrophysiol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.; Gupta, D. Left atrial posterior wall isolation—The conundrum of safety versus efficacy. J. Cardiovasc. Electrophysiol. 2022, 33, 1675–1677. [Google Scholar] [CrossRef]
- Müller, P.; Dietrich, J.-W.; Halbfass, P.; Abouarab, A.; Fochler, F.; Szöllösi, A.; Nentwich, K.; Roos, M.; Krug, J.; Schade, A.; et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm. 2015, 12, 1464–1469. [Google Scholar] [CrossRef]
- Cordes, F.; Ellermann, C.; Dechering, D.G.; Lange, P.S.; Pott, C.; Frommeyer, G.; Kochha, S.; Lenze, F.; Schmidt, H.; Ullerich, H.; et al. Time-to-isolation-guided cryoballoon ablation reduces oesophageal and mediastinal alterations detected by endoscopic ultrasound: Results of the MADE-PVI trial. EP Eur. 2019, 21, 1325–1333. [Google Scholar] [CrossRef]
- Baqal, O.; El Masry, H.Z. Ablative Management of Persistent Atrial Fibrillation (PeAF) with Posterior Wall Isolation (PWI): Where Do We Stand? J. Cardiovasc. Dev. Dis. 2023, 10, 273. [Google Scholar] [CrossRef]
- Singh, S.; Goel, S.; Chaudhary, R.; Garg, A.; Tantry, U.S.; Gurbel, P.A.; Garg, L. Safety and Effectiveness of Adjunctive Posterior Wall Isolation in Atrial Fibrillation Patients: An Updated Meta-Analysis. Am. J. Cardiol. 2023, 203, 64–72. [Google Scholar] [CrossRef]
- Hsia, H.H.; Xie, Y. Catheter Ablation in Scar: A Journey Into the Unknown. JACC Clin. Electrophysiol. 2019, 5, 932–934. [Google Scholar] [CrossRef]
- Younis, A.; Zilberman, I.; Krywanczyk, A.; Higuchi, K.; Yavin, H.D.; Sroubek, J.; Anter, E. Effect of Pulsed-Field and Radiofrequency Ablation on Heterogeneous Ventricular Scar in a Swine Model of Healed Myocardial Infarction. Circ. Arrhythm. Electrophysiol. 2022, 15, e011209. [Google Scholar] [CrossRef]
- Kawamura, I.; Reddy, V.Y.; Wang, B.J.; Dukkipati, S.R.; Chaudhry, H.W.; Santos-Gallego, C.G.; Koruth, J.S. Pulsed Field Ablation of the Porcine Ventricle Using a Focal Lattice-Tip Catheter. Circ. Arrhythm. Electrophysiol. 2022, 15, e011120. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Reddy, V.Y.; Santos-Gallego, C.G.; Wang, B.J.; Chaudhry, H.W.; Buck, E.D.; Mavroudis, G.; Jerrell, S.; Schneider, C.W.; Speltz, M.; et al. Electrophysiology, Pathology, and Imaging of Pulsed Field Ablation of Scarred and Healthy Ventricles in Swine. Circ. Arrhythm. Electrophysiol. 2023, 16, e011369. [Google Scholar] [CrossRef] [PubMed]
- Tohoku, S.; Chun, K.R.J.; Bordignon, S.; Chen, S.; Schaack, D.; Urbanek, L.; Ebrahimi, R.; Hirokami, J.; Bologna, F.; Schmidt, B. Findings from repeat ablation using high-density mapping after pulmonary vein isolation with pulsed field ablation. Europace 2022, 25, 433–440. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef] [PubMed]





| LAPWI n = 59 | |
|---|---|
| Age (years) | 64 ± 14 |
| Male gender, n (%) | 46 (78) |
| Hypertension, n (%) | 37 (63) |
| BMI (kg/m2) | 28 ± 4 |
| CHA2DS2-VASc score | 2 [1–4] |
| Persistent AF, n (%) | 59 (100) |
| History of AF (years) | 8 (3–13) |
| Prior AAD, n (%) | 31 |
| 20 |
| 11 |
| Prior catheter ablation procedures (n = 43) * | 2 (2–3) [max. 7] |
| Left atrial diameter (mm) | 45 (42–50) |
| Left ventricular function, n (%) | |
| 44 (75) 5 (8) 6 (10) 4 (7) |
| All n = 59 | |
|---|---|
| Procedural Data | |
| Initial Rhythm | |
| 29 (49) |
| 29 (49) |
| 1 (2) * |
| Electrical cardioversion prior to ablation (only in the patients presenting in AF/AT, n (%) | 23 (77) |
| Total procedure time (min) | 91 ± 30 |
| Total fluoroscopy time (min) | 14 ± 7 |
Three-dimensional mapping, n (%)
| 59 (100) 39 (66) |
| Patients with visualization of PFA catheter in mapping system, n (%) | 59 (100) |
| Pulsed Field ablation | |
| Time of PFA catheter in the left atrium (min) | 44 ± 17 |
| Fluoroscopy time during PFA (min) | 9 ± 5 |
| Total PFA applications per PV per patient | |
| LSPV | 8 [8–8] |
| LIPV | 8 [8–8] |
| RSPV | 8 [8–8] |
| RIPV | 8 [8–8] |
| Additional PFA applications | |
| LAPWI (n = 59) | 19 [10–26] |
| Mitral isthmus line (n = 6) Anterior line (n = 9) Inferior LA (n = 1) | 8 [4–11] 10 [7–18] 10 |
| PFA catheter size | |
| 31 mm 35 mm | 50 (85) 9 (15) |
| Additional RF ablation | |
| CTI ablation | 3 (5) |
| All complications, n (%) | 2 (3.4) |
| 0 (0.0) |
| 0 (0.0) |
| 0 (0.0) |
| 0 (0.0) |
| 1 (0.0) |
| 1 (0.0) |
| Baseline Data | PVI Only n = 16 | Comparison to LAPWI Cohort p-Value |
|---|---|---|
| Age (years) | 69 ± 9 | 0.2 |
| Male gender, n (%) | 10 (63) | 0.2 |
| Hypertension, n (%) | 12 (75) | 0.6 |
| BMI (kg/m2) | 26 ± 5 | 0.4 |
| CHA2DS2-VASc score | 2 [1–4] | 0.5 |
| Persistent AF, n (%) | 16 (100) | 0.1 |
| History of AF (years) | 2 [0.5–6] | 0.18 |
| Prior AAD, n (%) | 3 (19) | 0.02 |
| 0 (0) | |
| 3 (19) | |
| Prior catheter ablation procedures, n (%) | 0 (0%) | <0.001 |
| Left atrial diameter (mm) | 44 (40–46) | 0.4 |
| Left ventricular function, n (%) | ||
| 14 (88) 1 (6) 1 (6) 0 (0) | 0.3 |
| Procedural data | ||
| Total procedure time (min) | 76 ± 31 | 0.095 |
| Total fluoroscopy time (min) | 14 ± 7 | 0.94 |
| Three-dimensional mapping, n (%) High-density mapping, n (%) | 16 (100) 6 (38) | 0.1 |
| Total PFA applications per PV per patient | 0.12 | |
| LSPV | 8 [8–8] | |
| LIPV | 8 [8–8] | |
| RSPV | 8 [8–8.25] | |
| RIPV | 8 [8–8] | |
| PFA catheter size | ||
| 31 mm 35 mm | 14 (85) 2 (15) | 0.1 |
| Additional PFA ablation | 0 (0) | <0.001 |
| Additional RF ablation CTI ablation | 0 (0) 0 (0) | 0.1 |
| All complications, n (%) | 0 (0) | 0.1 |
| Pericardial tamponade, n (%) | 0 (0.0) | |
| Stroke/TIA | 0 (0.0) | |
| Phrenic nerve palsy, n (%) | 0 (0.0) | |
| Esophageal lesion, n (%) | 0 (0.0) | |
| Coronary spasm, n (%) | 0 (0.0) | |
| Minor groin complications, n (%) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunawardene, M.A.; Frommeyer, G.; Ellermann, C.; Jularic, M.; Leitz, P.; Hartmann, J.; Lange, P.S.; Anwar, O.; Rath, B.; Wahedi, R.; et al. Left Atrial Posterior Wall Isolation with Pulsed Field Ablation in Persistent Atrial Fibrillation. J. Clin. Med. 2023, 12, 6304. https://doi.org/10.3390/jcm12196304
Gunawardene MA, Frommeyer G, Ellermann C, Jularic M, Leitz P, Hartmann J, Lange PS, Anwar O, Rath B, Wahedi R, et al. Left Atrial Posterior Wall Isolation with Pulsed Field Ablation in Persistent Atrial Fibrillation. Journal of Clinical Medicine. 2023; 12(19):6304. https://doi.org/10.3390/jcm12196304
Chicago/Turabian StyleGunawardene, Melanie A., Gerrit Frommeyer, Christian Ellermann, Mario Jularic, Patrick Leitz, Jens Hartmann, Philipp Sebastian Lange, Omar Anwar, Benjamin Rath, Rahin Wahedi, and et al. 2023. "Left Atrial Posterior Wall Isolation with Pulsed Field Ablation in Persistent Atrial Fibrillation" Journal of Clinical Medicine 12, no. 19: 6304. https://doi.org/10.3390/jcm12196304
APA StyleGunawardene, M. A., Frommeyer, G., Ellermann, C., Jularic, M., Leitz, P., Hartmann, J., Lange, P. S., Anwar, O., Rath, B., Wahedi, R., Eckardt, L., & Willems, S. (2023). Left Atrial Posterior Wall Isolation with Pulsed Field Ablation in Persistent Atrial Fibrillation. Journal of Clinical Medicine, 12(19), 6304. https://doi.org/10.3390/jcm12196304