Low-Dose Rivaroxaban to Prevent Recurrences of Venous Thromboembolism in Cancer: A Real-Life Experience with a Focus on Female Patients
Abstract
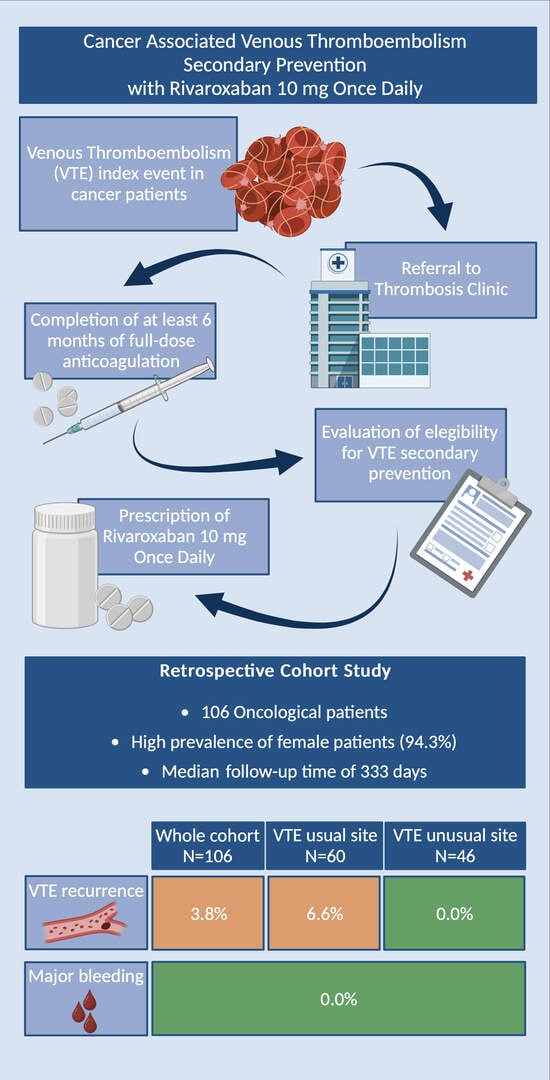
:1. Background
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girardi, L.; Wang, T.F.; Ageno, W.; Carrier, M. Updates in the Incidence, Pathogenesis, and Management of Cancer and Venous Thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 824–831. [Google Scholar] [CrossRef]
- Moik, F.; Colling, M.; Mahé, I.; Jara-Palomares, L.; Pabinger, I.; Ay, C. Extended anticoagulation treatment for cancer-associated thrombosis-Rates of recurrence and bleeding beyond 6 months: A systematic review. J. Thromb. Haemost. 2022, 20, 619–634. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef]
- Mahé, I.; Agnelli, G.; Ay, C.; Bamias, A.; Becattini, C.; Carrier, M.; Chapelle, C.; Cohen, A.T.; Girard, P.; Huisman, M.V.; et al. Extended Anticoagulant Treatment with Full- or Reduced-Dose Apixaban in Patients with Cancer-Associated Venous Thromboembolism: Rationale and Design of the API-CAT Study. Thromb. Haemost. 2022, 122, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Garresori, H.; Brekke, J.; Enden, T.; Frøen, H.; Jacobsen, E.M.; Quist-Paulsen, P.; Porojnicu, A.C.; Ree, A.H.; Torfoss, D.; et al. Low dose apixaban as secondary prophylaxis of venous thromboembolism in cancer patients—30 months follow-up. J. Thromb. Haemost. 2022, 20, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.; Noble, S.; Lee, A.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S.; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I.; Amplify-Ext Investigators. Apixaban for extended treatment of venous thromboembolism. N. Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [CrossRef]
- Weitz, J.I.; Lensing, A.W.; Prins, M.H.; Bauersachs, R.; Beyer-Westendorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N. Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef]
- Giustozzi, M.; Franco, L.; Agnelli, G.; Verso, M. Unmet clinical needs in the prevention and treatment of cancer-associated venous thromboembolism. Trends Cardiovasc. Med. 2022, 33, 336–343. [Google Scholar] [CrossRef]
- Yamashita, Y.; Morimoto, T.; Amano, H.; Takase, T.; Hiramori, S.; Kim, K.; Konishi, T.; Akao, M.; Kobayashi, Y.; Inoue, T.; et al. Anticoagulation Therapy for Venous Thromboembolism in the Real World—From the COMMAND VTE Registry. Circ. J. 2018, 82, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Lapébie, F.X.; Bura-Rivière, A.; Merah, A.; Bertoletti, L.; Monreal, M.; RIETE Investigators. OC-15 Risk factors of recurrence in cancer-associated venous thromboembolism after discontinuation of anticoagulant therapy: A RIETE-based prospective study. Thromb. Res. 2021, 200 (Suppl. 1), S15. [Google Scholar] [CrossRef]
- Francis, C.W.; Kessler, C.M.; Goldhaber, S.Z.; Kovacs, M.J.; Monreal, M.; Huisman, M.V.; Bergqvist, D.; Turpie, A.G.; Ortel, T.L.; Spyropoulos, A.C.; et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: The DALTECAN Study. J. Thromb. Haemost. 2015, 13, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palomares, L.; Solier-Lopez, A.; Elias-Hernandez, T.; Asensio-Cruz, M.; Blasco-Esquivias, I.; Marin-Barrera, L.; de la Borbolla-Artacho, M.R.; Praena-Fernandez, J.M.; Montero-Romero, E.; Navarro-Herrero, S.; et al. Tinzaparin in cancer associated thrombosis beyond 6months: TiCAT study. Thromb. Res. 2017, 157, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Cote, L.P.; Greenberg, S.; Caprini, J.A.; Tafur, A.; Choi, C.; Muñoz, F.J.; Skride, A.; Valero, B.; Porras, J.A.; Ciammaichella, M.; et al. Comparisons Between Upper and Lower Extremity Deep Vein Thrombosis: A Review of the RIETE Registry. Clin. Appl. Thromb. Hemost. 2017, 23, 748–754. [Google Scholar] [CrossRef]
- Thiyagarajah, K.; Ellingwood, L.; Endres, K.; Hegazi, A.; Radford, J.; Iansavitchene, A.; Lazo-Langner, A. Post-thrombotic syndrome and recurrent thromboembolism in patients with upper extremity deep vein thrombosis: A systematic review and meta-analysis. Thromb. Res. 2019, 174, 34–39. [Google Scholar] [CrossRef]
- Valeriani, E.; Di Nisio, M.; Porceddu, E.; Agostini, F.; Pola, R.; Spoto, S.; Donadini, M.P.; Ageno, W.; Porfidia, A. Anticoagulant treatment for upper extremity deep vein thrombosis: A systematic review and meta-analysis. Thromb. Haemost. 2022, 20, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Frere, C.; Crichi, B.; Lejeune, M.; Spano, J.P.; Janus, N. Are Patients with Active Cancer and Those with History of Cancer Carrying the Same Risks of Recurrent VTE and Bleeding While on Anticoagulants? Cancers 2020, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.H.; Lensing, A.W.; Bauersachs, R.; van Bellen, B.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; Raskob, G.E.; et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: A pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb. J. 2013, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; van Es, N.; Segers, A.; Angchaisuksiri, P.; Oh, D.; Boda, Z.; Lyons, R.M.; Meijer, K.; Gudz, I.; Weitz, J.I.; et al. Edoxaban for venous thromboembolism in patients with cancer: Results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol. 2016, 3, e379–e387. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 106) |
|---|---|
| Male/female ratio | 6/100 |
| Median age, yrs (IQR) | 60 (50–69) |
| Cancer activity, n (%) | |
| 94 (88.7) |
| 12 (11.3) |
| Active oncological treatment | 89 (84.0) |
| 63 (59.4) |
| 26 (24.5) |
| 29 (27.3) |
| 16 (15.1) |
| 18 (17.0) |
| Cancer stage, n (%) | |
| 25 (23.6) |
| 81 (76.4) |
| 6 (5.7) |
| Primary cancer site, n (%) | |
| 31 (29.2) |
| 43 (40.6) |
| 17 (16.0) |
| 7 (6.6) |
| 5 (4.7) |
| 1 (0.9) |
| 1 (0.9) |
| 1 (0.9) |
| 1 (0.9) |
| 1 (0.9) |
| 1 (0.9) |
| 1 (0.9) |
| 4 (3.8) |
| Type of VTE index event, n (%) | |
| 60 (56.6) |
| 46 (43.4) |
| VTE index event site, n (%) | |
| 39 (36.8) |
| 21 (19.8) |
| 43 (40.6) |
| 3 (2.8) |
| Previous history of VTE, n (%) | 9 (8.5) |
| Comorbidities, n (%) | |
| 50 (47.2) |
| 21 (19.8) |
| 10 (9.4) |
| 3 (2.8) |
| 1 (0.9) |
| Creatinine clearance < 50 mL/min, n (%) | 26 (24.7) |
| Median follow-up time on low-dose rivaroxaban, days (IQR) | 333 (156–484) |
| Outcomes | Whole Cohort | Patients with Index Event in Usual Site | Patients with Index Event in Upper Limbs |
|---|---|---|---|
| Effectiveness | |||
| VTE recurrences, n/total (%) | 4/106 (3.8) | 4/60 (6.6) | 0/46 (0.0) |
| VTE recurrence rate, n per 100 persons per year | 4.0 | 7.5 | 0.0 |
| Type of VTE recurrence | |||
| 0/4 (0.0) | 0/4 (0.0) | 0/4 (0.0) |
| 4/4 (100.0) | 4/4 (100.0) | 0/4 (0.0) |
| Safety | |||
| MB, n/total (%) | 0.0 | 0.0 | 0.0 |
| CRNMB, n/total (%) | 3/106 (2.8) | 2/60 (3.3) | 1/46 (2.2) |
| Site of CRNMB | |||
| 1/3 (33.3) | 1/2 (50.0) | 0/1 (0.0) |
| 1/3 (33.3) | 1/2 (50.0) | 0/1 (0.0) |
| 1/3 (33.3) | 0/2 (0.0) | 1/1 (100.0) |
| All-cause mortality rate, n per 100 persons per year | 11.0 | 13.2 | 8.5 |
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | |
|---|---|---|---|---|
| Age | 76 | 56 | 66 | 57 |
| Sex | Female | Female | Female | Male |
| BMI | 35.3 | 30.9 | 29.4 | 23.7 |
| Cancer activity | Active cancer | Active cancer | Active cancer | History of cancer |
| Cancer site | Ovarian | Endometrial | Ovarian | Tonsil |
| Cancer stage | Metastatic | Metastatic | Metastatic | Localised |
| Active oncological treatment | Yes | Yes | Yes | No |
| VTE index event | PE and proximal DVT of lower limbs (May 2021) | PE (August 2021) | PE and proximal DVT of lower limbs (September 2021) | Proximal DVT (February 2022) |
| Previous anticoagulant regimen (days) | Enoxaparin 6000 U.I. twice daily (209) | Enoxaparin 6000 U.I. twice daily (52) followed by edoxaban 60 mg once daily (128) | Enoxaparin 6000 U.I. twice daily (29) followed by apixaban 5 mg twice daily (165) | Enoxaparin 6000 UI twice daily (71) followed by edoxaban 60 mg once daily (116) |
| Days from VTE to dose reduction | 215 | 180 | 194 | 187 |
| VTE recurrence | Proximal DVT | Proximal DVT | Proximal DVT | Proximal DVT |
| Days from dose reduction to VTE recurrence | 292 | 130 | 104 | 248 |
| Extrinsic vascular compression | Yes | No | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santini, P.; Mosoni, C.; D’Errico, A.; Porceddu, E.; Lupascu, A.; Valeriani, E.; Tondi, P.; Pola, R.; Porfidia, A. Low-Dose Rivaroxaban to Prevent Recurrences of Venous Thromboembolism in Cancer: A Real-Life Experience with a Focus on Female Patients. J. Clin. Med. 2023, 12, 6427. https://doi.org/10.3390/jcm12196427
Santini P, Mosoni C, D’Errico A, Porceddu E, Lupascu A, Valeriani E, Tondi P, Pola R, Porfidia A. Low-Dose Rivaroxaban to Prevent Recurrences of Venous Thromboembolism in Cancer: A Real-Life Experience with a Focus on Female Patients. Journal of Clinical Medicine. 2023; 12(19):6427. https://doi.org/10.3390/jcm12196427
Chicago/Turabian StyleSantini, Paolo, Carolina Mosoni, Alessandro D’Errico, Enrica Porceddu, Andrea Lupascu, Emanuele Valeriani, Paolo Tondi, Roberto Pola, and Angelo Porfidia. 2023. "Low-Dose Rivaroxaban to Prevent Recurrences of Venous Thromboembolism in Cancer: A Real-Life Experience with a Focus on Female Patients" Journal of Clinical Medicine 12, no. 19: 6427. https://doi.org/10.3390/jcm12196427
APA StyleSantini, P., Mosoni, C., D’Errico, A., Porceddu, E., Lupascu, A., Valeriani, E., Tondi, P., Pola, R., & Porfidia, A. (2023). Low-Dose Rivaroxaban to Prevent Recurrences of Venous Thromboembolism in Cancer: A Real-Life Experience with a Focus on Female Patients. Journal of Clinical Medicine, 12(19), 6427. https://doi.org/10.3390/jcm12196427