From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions
Abstract
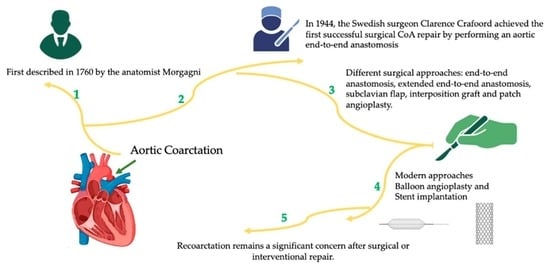
:1. Introduction
2. Methodology
3. Coarctation Management
3.1. Surgical Approach
3.1.1. Resection with End-to-End Anastomosis
3.1.2. Subclavian Flap Repair
3.1.3. Interposition Graft
3.1.4. Patch Angioplasty
3.1.5. Extended End-to-End Anastomosis
3.1.6. Single vs. Stage-Management Approach for Coarctation of the Aorta with Associated Congenital Cardiac Anomalies
3.2. Transcathether Interventions
3.2.1. Balloon Angioplasty
3.2.2. Stent Implantation
4. Comparative Analysis of Aortic Coarctation Repair Techniques
5. Long-Term Outcomes after Coarctation Repair
6. Recoarctation: Definitions and Thresholds for Reintervention
6.1. Contemporany Definition of Recoarctation
6.2. Thresolds for Reintervention
7. Our Experience-Case Series
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raza, S.; Aggarwal, S.; Jenkins, P.; Kharabish, A.; Anwer, S.; Cullington, D.; Jones, J.; Dua, J.; Papaioannou, V.; Ashrafi, R.; et al. Coarctation of the Aorta: Diagnosis and Management. Diagnostics 2023, 13, 2189. [Google Scholar] [CrossRef]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; De Simone, G.; Ford, E.S.; et al. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of Congenital Heart Defects in Metropolitan Atlanta, 1998–2005. J. Pediatr. 2008, 153, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.W. Congenital heart disease in the newborn requiring early intervention. Korean J. Pediatr. 2011, 54, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Crafoord, C.; Nylin, G. Congenital coarctation of the aorta and its surgical treatment. J. Thorac. Surg. 1945, 14, 347. [Google Scholar] [CrossRef]
- Kvitting, J.P.; Olin, C.L. Clarence Crafoord: A giant in cardiothoracic surgery, the first to repair aortic coarctation. Ann. Thorac. Surg. 2009, 87, 342–346. [Google Scholar] [CrossRef]
- Gross, R.E. Surgical correction for coarctation of the aorta. Surgery 1945, 18, 673–678. [Google Scholar] [PubMed]
- Ungerleider, R.M.; Pasquali, S.K.; Welke, K.F.; Wallace, A.S.; Ootaki, Y.; Quartermain, M.D.; Williams, D.A.; Jacobs, J.P. Contemporary patterns of surgery and outcomes for aortic coarctation: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J. Thorac. Cardiovasc. Surg. 2013, 145, 150–158. [Google Scholar] [CrossRef]
- Gross, R.; Hufnagel, C. Coarctation of the aorta. Experimental studies regarding its surgical correction. N. Engl. J. Med. 1945, 233, 287–293. [Google Scholar] [CrossRef]
- Williams, W.G.; Shindo, G.; Trusler, G.A.; Dische, M.R.; Olley, P.M. Results of repair of coarctation of the aorta during infancy. J. Thorac. Cardiovasc. Surg. 1980, 79, 603–608. [Google Scholar] [CrossRef]
- Backer, C.L.; Mavroudis, C.; Zias, E.A.; Amin, Z.; Weigel, T.J. Repair of coarctation with resection and extended end-to-end anastomosis. Ann. Thorac. Surg. 1998, 66, 1365–1370, discussion 1370-1. [Google Scholar] [CrossRef] [PubMed]
- Hesslein, P.S.; McNamara, D.G.; Morriss, M.J.; Hallman, G.L.; Cooley, D.A. Comparison of resection versus partch aortoplasty for repair of coarctation in infants and children. Circulation 1981, 64, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Ziemer, G.; Jonas, R.A.; Perry, S.B.; Freed, M.D.; Castaneda, A.R. Surgery for coarctation of the aorta in the neonate. Circulation 1986, 74, 25–31. [Google Scholar]
- Waldhausen, J.A.; Nahrwold, D. Repair of coarctation of the aorta with a subclavian flap. J. Thorac. Cardiovasc. Surg. 1966, 51, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.E.; Davidson, W.R.; Swallow, N.A.; Nickolaus, M.J.; Myers, J.L.; Clark, J.B. Long-Term Results of the Subclavian Flap Repair for Coarctation of the Aorta in Infants. World J. Pediatr. Congenit. Heart Surg. 2013, 4, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, C.J.; Ellison, T.A.; Williams, J.A.; Durr, M.L.; Cameron, D.E.; Vricella, L.A. Subclavian flap aortoplasty: Still a safe, reproducible, and effective treatment for infant coarctation. Eur. J. Cardiothorac. Surg. 2007, 31, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Beekman, R.H.; Rocchini, A.P.; Behrendt, D.M.; Bove, E.L.; Dick, M., II; Crowley, D.C.; Snider, A.R.; Rosenthal, A. Long-term outcome after repair of coarctation in infancy: Subclavian angioplasty does not reduce the need for reoperation. J. Am. Coll. Cardiol. 1986, 8, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Jackson, M.; Ajab, S.; Gladman, G.; Pozzi, M. Subclavian flap repair: Review of 399 patients at median follow-up of fourteen years. Ann. Thorac. Surg. 2006, 81, 1420–1428. [Google Scholar] [CrossRef]
- Gross, R.E. Treatment of certain aortic coarctations by homologous grafts; a report of nineteen cases. Ann. Surg. 1951, 134, 753–768. [Google Scholar] [CrossRef]
- Yousif, A.; Kloppenburg, G.; Morshuis, W.J.; Heijmen, R. Repair of adult aortic coarctation by resection and interposition grafting. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 526–530. [Google Scholar] [CrossRef]
- Charlton-Ouw, K.M.; Codreanu, M.E.; Leake, S.S.; Sandhu, H.K.; Calderon, D.; Azizzadeh, A.; Estrera, A.L.; Safi, H.J. Open repair of adult aortic coarctation mostly by a resection and graft replacement technique. J. Vasc. Surg. 2015, 61, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Vossschulte, K. Isthmusplastik zur Behandlung der Aortenisthmus- stenose. Thoraxchirurgie 1957, 4, 443–450. [Google Scholar] [PubMed]
- Venturini, A.; Perna, A.; Bianchi, G. Repair of coarctation of the thoracic aorta without resection. Patch graft aortoplasty. Follow-up study of 46 cases. J. Cardiovasc. Surg. 1978, 19, 49–54. [Google Scholar]
- Walhout, R.J.; Lekkerkerker, J.C.; Oron, G.H.; Hitchcock, F.J.; Meijboom, E.J.; Bennink, G.B. Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J. Thorac. Cardiovasc. Surg. 2003, 126, 521–528. [Google Scholar] [CrossRef]
- Amato, J.J.; Rheinlander, H.; Cleveland, R. A method of enlarging the distal transverse arch in infants with hypoplasia and coarctation of the aorta. Ann. Thorac. Surg. 1977, 23, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Ruzmetov, M.; Hoyer, M.H.; Rodefeld, M.D.; Turrentine, M.W. Recurrent Coarctation: Is Surgical Repair of Recurrent Coarctation of the Aorta Safe and Effective? Ann. Thorac. Surg. 2009, 88, 1923–1930, discussion 1930–1931. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.D.; Mulpur, A.; Guerrero, R.; Nagy, Z.; Gibbs, J.L.; Watterson, K.G. Outcome after extended arch repair for aortic coarctation. Heart 2006, 92, 90–94. [Google Scholar] [CrossRef]
- Hager, A.; Schreiber, C.; Nützl, S.; Hess, J. Mortality and restenosis rate of surgical coarctation repair in infancy: A study of 191 patients. Cardiology 2009, 112, 36–41. [Google Scholar] [CrossRef]
- Karamlou, T.; Bernasconi, A.; Jaeggi, E.; Alhabshan, F.; Williams, W.G.; Van Arsdell, G.S.; Coles, J.G.; Caldarone, C.A. Factors associated with arch reintervention and growth of the aortic arch after coarctation repair in neonates weighing less than 2.5 kg. J. Thorac. Cardiovasc. Surg. 2009, 137, 1163–1167. [Google Scholar] [CrossRef]
- Tabbutt, S.; Nicolson, S.C.; Dominguez, T.E.; Wells, W.; Backer, C.L.; Tweddell, J.S.; Bokesch, P.; Schreiner, M. Perioperative course in 118 infants and children undergoing coarctation repair via a thoracotomy: A prospective, multicenter experience. J. Thorac. Cardiovasc. Surg. 2008, 136, 1229–1236. [Google Scholar] [CrossRef]
- Wright, G.E.; Nowak, C.A.; Goldberg, C.S.; Ohye, R.G.; Bove, E.L.; Rocchini, A.P. Extended resection and end-to-end anastomosis for aortic coarctation in infants: Results of a tailored surgical approach. Ann. Thorac. Surg. 2005, 80, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Zurakowski, D.; Sharma, R.; Saini, S.; Jonas, R.A. Prediction of recurrent coarctation by early postoperative blood pressure gradient. J. Thorac. Cardiovasc. Surg. 2011, 142, 1130–1136.e1. [Google Scholar] [CrossRef] [PubMed]
- Akam-Venkata, J.; Ikemba, C.M.; Martinez, J.J.; Pruszynski, J.; Heistein, L.C.; Pirolli, T.; Forbess, J. Single-Stage Surgical Management of Atrioventricular Septal Defects with Coarctation of the Aorta. Pediatr. Cardiol. 2022, 43, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Kanter, K.R. Management of infants with coarctation and ventricular septal defect. Semin. Thorac. Cardiovasc. Surg. 2007, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.I.; Rowen, M.; Dorsey, T.J. Transluminal aortic balloon angioplasty for coarctation of the aorta in the newborn. Am. Heart J. 1982, 103, 131–132. [Google Scholar] [CrossRef]
- Doshi, A.R.; Syamasundar Rao, P. Coarctation of aorta-management options and decision making. Pediat Therapeut. 2012, 5, 6. [Google Scholar] [CrossRef]
- Tomar, M.; Radhakrishanan, S. Coarctation of aorta--intervention from neonates to adult life. Indian Heart J. 2008, 60 (Suppl SD), D22–D33. [Google Scholar]
- Fruh, S.; Knirsch, W.; Dodge-Khatami, A.; Dave, H.; Pretre, R.; Kretschmar, O. Comparison of surgical and interventional therapy of native and recurrent aortic coarctation regarding different age groups during childhood. Eur. J. Cardiothorac. Surg. 2011, 39, 898–904. [Google Scholar] [CrossRef]
- Garg, G.; Goyal, N.; Mandhan, G.; Sidana, P. Transfemoral balloon angioplasty of severe coarctation of aorta in 1200 g newborn. Ann. Pediatr. Cardiol. 2017, 10, 95–96. [Google Scholar] [CrossRef]
- Moustafa, G.A.; Kolokythas, A.; Charitakis, K.; Avgerinos, D.V. Therapeutic Utilities of Pediatric Cardiac Catheterization. Curr. Cardiol. Rev. 2016, 12, 258–269. [Google Scholar] [CrossRef]
- Fiore, A.C.; Fischer, L.K.; Schwartz, T.; Jureidini, S.; Balfour, I.; Carpenter, D.; Demello, D.; Virgo, K.S.; Pennington, D.G.; Johnson, R.G. Comparison of angioplasty and surgery for neonatal aortic coarctation. Ann. Thorac. Surg. 2005, 80, 1659–1664, discussion 1664—1665. [Google Scholar] [CrossRef]
- Bouzguenda, I.; Marini, D.; Ou, P.; Boudjemline, Y.; Bonnet, D.; Agnoletti, G. Percutaneous treatment of neonatal aortic coarctation presenting with severe left ventricular dysfunction as a bridge to surgery. Cardiol. Young 2009, 19, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Meliota, G.; Lombardi, M.; Zaza, P.; Tagliente, M.R.; Vairo, U. Balloon angioplasty of aortic coarctation in critically ill newborns using axillary artery access. Ann. Pediatr. Cardiol. 2020, 13, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.; Galindo, A.; Evans, W.N.; Collazos, J.C.; Restrepo, H. Effectiveness and safety of balloon dilation of native aortic coarctation in premature neonates weighing < or =2500 grams. Am. J. Cardiol. 2010, 105, 1176–1180. [Google Scholar] [PubMed]
- Sandoval, J.P.; Kang, S.L.; Lee, K.J.; Benson, L.; Asoh, K.; Chaturvedi, R.R. Balloon Angioplasty for Native Aortic Coarctation in 3- to 12-Month-Old Infants. Circ. Cardiovasc. Interv. 2020, 13, e008938. [Google Scholar] [CrossRef]
- O’Laughlin, M.P.; Perry, S.B.; Lock, J.E.; Mullins, C.E. Use of endovascular stents in congenital heart disease. Circulation 1991, 83, 1923–1939. [Google Scholar] [CrossRef]
- Egan, M.; Holzer, R.J. Comparing balloon angioplasty, stenting and surgery in the treatment of aortic coarctation. Expert Rev. Cardiovasc. Ther. 2009, 7, 1401–1412. [Google Scholar] [CrossRef]
- Doshi, A.R.; Rao, P.S. Development of aortic coarctation following device closure of patent ductus arteriosus. J. Invasive Cardiol. 2013, 25, 464–467. [Google Scholar]
- Gewillig, M.; Budts, W.; Boshoff, D.; Maleux, G. Percutaneous interventions of the aorta. Future Cardiol. 2012, 8, 251–269. [Google Scholar] [CrossRef]
- Cardoso, G.; Abecasis, M.; Anjos, R.; Marques, M.; Koukoulis, G.; Aguiar, C.; Neves, J.P. Aortic coarctation repair in the adult. J. Card. Surg. 2014, 29, 512–518. [Google Scholar] [CrossRef]
- Suárez de Lezo, J.; Romero, M.; Pan, M.; Suárez de Lezo, J.; Segura, J.; Ojeda, S.; Pavlovic, D.; Mazuelos, F.; López Aguilera, J.; Espejo Perez, S. Stent Repair for Complex Coarctation of Aorta. JACC Cardiovasc. Interv. 2015, 8, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Dijkema, E.J.; Leiner, T.; Grotenhuis, H.B. Diagnosis, imaging and clinical management of aortic coarctation. Heart 2017, 103, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Forbes, T.J.; Kim, D.W.; Du, W.; Turner, D.R.; Holzer, R.; Amin, Z.; Hijazi, Z.; Ghasemi, A.; Rome, J.J.; Nykanen, D. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: An observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). J. Am. Coll Cardiol. 2011, 58, 2664–2674. [Google Scholar] [CrossRef]
- Cohen, M.; Fuster, V.; Steele, P.M.; Driscoll, D.; McGoon, D.C. Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction. Circulation 1989, 80, 840–845. [Google Scholar] [CrossRef]
- Brown, M.L.; Burkhart, H.M.; Connolly, H.M.; Dearani, J.A.; Cetta, F.; Li, Z.; Oliver, W.C.; Warnes, C.A.; Schaff, H.V. Coarctation of the aorta: Lifelong surveillance is mandatory following surgical repair. J. Am. Coll. Cardiol. 2013, 62, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, R.; Breur, J.M.P.J.; Heuser, J.; Jansen, N.J.G.; de Vries, W.B.; Vijlbrief, D.C.; Molenschot, M.M.C.; Haas, F.; Krings, G.J. Primary coronary stent implantation is a feasible bridging therapy to surgery in very low birth weight infants with critical aortic coarctation. Int. J. Cardiol. 2018, 261, 62–65. [Google Scholar] [CrossRef]
- Mini, N.; Zartner, P.A.; Schneider, M.B.E. Stenting of critical aortic coarctation in neonates between 600 and 1350 g. Using a transfemoral artery approach: A single center experience. Front. Cardiovasc. Med. 2022, 9, 1025411. [Google Scholar] [CrossRef]
- Gorenflo, M.; Boshoff, D.E.; Heying, R.; Eyskens, B.; Rega, F.; Meyns, B.; Gewillig, M. Bailout stenting for critical coarctation in premature/critical/complex/early recoarcted neonates. Catheter. Cardiovasc. Interv. 2009, 75, 553–561. [Google Scholar] [CrossRef]
- Sallmon, H.; Berger, F.; Cho, M.Y.; Opgen-Rhein, B. First use and limitations of Magmaris® bioresorbable stenting in a low birth weight infant with native aortic coarctation. Catheter. Cardiovasc. Interv. 2019, 93, 1340–1343. [Google Scholar] [CrossRef]
- Torok, R.D.; Campbell, M.J.; Fleming, G.A.; Hill, K.D. Coarctation of the aorta: Management from infancy to adulthood. World J. Cardiol. 2015, 7, 765–775. [Google Scholar] [CrossRef]
- Warnes, C.A.; Williams, R.G.; Bashore, T.M.; Child, J.S.; Connolly, H.M.; Dearani, J.A.; del Nido, P.; Fasules, J.W.; Graham, T.P.; Hijazi, Z.M.; et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008, 118, e714–e833. [Google Scholar] [CrossRef]
- Harris, K.C.; Du, W.; Cowley, C.G.; Forbes, T.J.; Kim, D.W. A prospective observational multicenter study of balloon angioplasty for the treatment of native and recurrent coarctation of the aorta. Catheter Cardiovasc. Interv. 2014, 83, 1116–1123. [Google Scholar] [CrossRef]
- Cowley, C.G.; Orsmond, G.S.; Feola, P.; McQuillan, L.; Shaddy, R.E. Long-term, randomized comparison of balloon angioplasty and surgery for native coarctation of the aorta in childhood. Circulation 2005, 111, 3453–3456. [Google Scholar] [CrossRef] [PubMed]
- Feltes, T.F.; Bacha, E.; Beekman, R.H.; Cheatham, J.P.; Feinstein, J.A.; Gomes, A.S.; Hijazi, Z.M.; Ing, F.F.; de Moor, M.; Morrow, W.R.; et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2607–2652. [Google Scholar] [CrossRef] [PubMed]
- Kische, S.; D’Ancona, G.; Stoeckicht, Y.; Ortak, J.; Elsässer, A.; Ince, H. Percutaneous treatment of adult isthmic aortic coarctation: Acute and long-term clinical and imaging outcome with a self-expandable uncovered nitinol stent. Circ. Cardiovasc. Interv. 2015, 8, e001799. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Zwinderman, A.H.; Bogers, A.J.; Rohmer, J.; Huysmans, H.A. More than thirty-five years of coarctation repair. An unexpected high relapse rate. J. Thorac. Cardiovasc. Surg. 1994, 107, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dehaki, M.G.; Ghavidel, A.A.; Givtaj, N.; Omrani, G.; Salehi, S. Recurrence rate of different techniques for repair of coarctation of aorta: A 10 years experience. Ann. Pediatr. Cardiol. 2010, 3, 123–126. [Google Scholar] [CrossRef]
- Uğuz, E.; Özkan, S.; Akay, H.T.; Gültekin, B.; Aşlamacı, S. Surgical repair of coarctation of aorta in neonates and infants: A 10 years experience. Turk. Gogus. Kalp. Dama 2010, 18, 1094–1099. [Google Scholar]
- Jahangiri, M.; Shinebourne, E.A.; Zurakowski, D.; Rigby, M.L.; Redington, A.N.; Lincoln, C. Subclavian flap angioplasty: Does the arch look after itself? J. Thorac. Cardiovasc. Surg. 2000, 120, 224–229. [Google Scholar] [CrossRef]
- Burch, P.T.; Cowley, C.G.; Holubkov, R.; Null, D.; Lambert, L.M.; Kouretas, P.C.; Hawkins, J.A. Coarctation repair in neonates and young infants: Is small size or low weight still a risk factor? J. Thorac. Cardiovasc. Surg. 2009, 138, 547–552. [Google Scholar] [CrossRef]
- Sen, S.; Garg, S.; Rao, S.G.; Kulkarni, S. Native aortic coarctation in neonates and infants: Immediate and midterm outcomes with balloon angioplasty and surgery. Ann. Pediatr. Cardiol. 2018, 11, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Chopra, P.S.; Koscik, R.; Smith, P.A.; Wilson, A.D. Surgical versus balloon therapy for aortic coarctation in infants < or = 3 months old. J. Am. Coll. Cardiol. 1994, 23, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Mohan, U.R.; Danon, S.; Levi, D.; Connolly, D.; Moore, J.W. Stent implantation for coarctation of the aorta in children <30 kg. JACC Cardiovasc. Interv. 2009, 2, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Corno, A.F.; Botta, U.; Hurni, M.; Payot, M.; Sekarski, N.; Tozzi, P.; von Segesser, L.K. Surgery for aortic coarctation: A 30 years experience. Eur. J. Cardiothorac. Surg. 2001, 20, 1202–1206. [Google Scholar] [CrossRef]
- Padalino, M.A.; Bagatin, C.; Bordin, G.; Tua, L.; Francescato, A.; Pradegan, N.; Piperata, A.; Vida, V.L.; Castaldi, B.; Boccuzzo, G.; et al. Surgical repair of aortic coarctation in pediatric age: A single center two decades experience. J. Card. Surg. 2019, 34, 256–265. [Google Scholar] [CrossRef]
- Kaushal, S.; Backer, C.L.; Patel, J.N.; Patel, S.K.; Walker, B.L.; Weigel, T.J.; Randolph, G.; Wax, D.; Mavroudis, C. Coarctation of the aorta: Midterm outcomes of resection with extended end-to-end anastomosis. Ann. Thorac. Surg. 2009, 88, 1932–1938. [Google Scholar] [CrossRef]
- Presbitero, P.; Demarie, D.; Villani, M.; Perinetto, E.A.; Riva, G.; Orzan, F.; Bobbio, M.; Morea, M.; Brusca, A. Long term results (15–30 years) of surgical repair of aortic coarctation. Br. Heart J. 1987, 57, 462–467. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Miró, J.; Dancea, A.; Ibrahim, R.; Piette, E.; Lapierre, C.; Jutras, L.; Perron, J.; Tchervenkow, C.I.; Poirier, N.; et al. Comparison of surgical and transcatheter treatment for native coarctation of the aorta in patients > or = 1 year old. The Quebec Native Coarctation of the Aorta study. Am. Heart J. 2007, 154, 186–192. [Google Scholar] [CrossRef]
- Raissadati, A.; Nieminen, H.; Haukka, J.; Sairanen, H.; Jokinen, E. Late Causes of Death After Pediatric Cardiac Surgery: A 60-Year Population-Based Study. J. Am. Coll. Cardiol. 2016, 68, 487–498. [Google Scholar] [CrossRef]
- Lee, M.G.Y.; Babu-Narayan, S.V.; Kempny, A.; Uebing, A.; Montanaro, C.; Shore, D.F.; d’Udekem, Y.; Gatzoulis, M.A. Long-term mortality and cardiovascular burden for adult survivors of coarctation of the aorta. Heart 2019, 105, 1190–1196. [Google Scholar] [CrossRef]
- Egbe, A.C.; Miranda, W.R.; Warnes, C.A.; Bonnichsen, C.; Crestanello, J.; Anderson, J.H.; Connolly, H.M. Persistent Hypertension and Left Ventricular Hypertrophy After Repair of Native Coarctation of Aorta in Adults. Hypertension 2021, 78, 672–680. [Google Scholar] [CrossRef]
- Choudhary, P.; Canniffe, C.; Jackson, D.J.; Tanous, D.; Walsh, K.; Celermajer, D.S. Late outcomes in adults with coarctation of the aorta. Heart 2015, 101, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- de Divitiis, M.; Pilla, C.; Kattenhorn, M.; Zadinello, M.; Donald, A.; Leeson, P.; Wallace, S.; Redington, A.; Deanfield, J.E. Vascular dysfunction after repair of coarctation of the aorta: Impact of Early Surgery. Circulation 2001, 104 (Suppl. S1), I-165–I-170. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Humphries, J.O.; Rowe, R.D.; Mellits, E.D. Prognosis of Surgically Corrected Coarctation of the Aorta: A 20-Year Postoperative Appraisal. Circulation 1973, 47, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Toro-Salazar, O.H.; Steinberger, J.; Thomas, W.; Rocchini, A.P.; Carpenter, B.; Moller, J.H. Long-term follow-up of patients after coarctation of the aorta repair. Am. J. Cardiol. 2002, 89, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Miranda, W.R.; Jain, C.C.; Borlaug, B.A.; Connolly, H.M. Prognostic Implications of Exercise-Induced Hypertension in Adults With Repaired Coarctation of Aorta. Hypertension 2022, 79, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Bonnet, D.; Auriacombe, L.; Pedroni, E.; Balleux, F.; Sidi, D.; Mousseaux, E. Late systemic hypertension and aortic arch geometry after successful repair of coarctation of the aorta. Eur. Heart J. 2004, 25, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, V.; Connolly, H.M.; Al-Otaibi, M.; Ammash, N.M.; Warnes, C.A.; Said, S.M.; Egbe, A.C. Prognostic Role of Hypertensive Response to Exercise in Patients with Repaired Coarctation of Aorta. Can. J. Cardiol. 2018, 34, 676–682. [Google Scholar] [CrossRef]
- Buys, R.; Van De Bruaene, A.; Müller, J.; Hager, A.; Khambadkone, S.; Giardini, A.; Cornelissen, V.; Budts, W.; Vanhees, L. Usefulness of cardiopulmonary exercise testing to predict the development of arterial hypertension in adult patients with repaired isolated coarctation of the aorta. Int. J. Cardiol. 2013, 168, 2037–2041. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1494–1563. [Google Scholar] [CrossRef]
- de Divitiis, M.; Pilla, C.; Kattenhorn, M.; Donald, A.; Zadinello, M.; Wallace, S.; Redington, A.; Deanfield, J. Ambulatory blood pressure, left ventricular mass, and conduit artery function late after successful repair of coarctation of the aorta. J. Am. Coll. Cardiol. 2003, 41, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, M.; Reinilä, A.; Tarkka, M.; Uhari, M. Reversibility of hypertensive vascular changes after coarctation repair in dogs. Pediatr. Res. 1992, 31, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Morgan, G.J.; Mitchell, M.B.; Ross, M.; Barker, A.J.; Hunter, K.S.; Fonseca, B.; DiMaria, M.; Vargas, D.; Ivy, D.D.; et al. Impact of different coarctation therapies on aortic stiffness: Phase-contrast MRI study. Int. J. Cardiovasc. Imaging 2018, 34, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.A.; Assef, J.E.; Pedra, S.R.; Ferreira, W.P.; Davoglio, T.A.; Petisco, A.C.; Saleh, M.H.; Le Bihan, D.C.; Barretto, R.B.; Pedra, C.A. Serial assessment of arterial structure and function in patients with coarctation of the aorta undergoing stenting. Int. J. Cardiovasc. Imaging 2016, 32, 729–739. [Google Scholar] [CrossRef]








| Study | Patients | Follow-Up | Recurrence Rate | Primary Repair Methods | Key Findings and Recoarctation Repair Approach |
|---|---|---|---|---|---|
| Kapetein et al. (1994) [66] | 109 | 30 years | 5.8% | Classic, Extended end-to-end | Classic repair had higher long-term recoarctation rate. Extended repair (polypropylene) showed no recoarctation. Age < 6 months was a prognostic factor. |
| Dehaki et al. (2010) [67] | 188 | 81.6 months | 10% | Patch, End-to-end, Subclavian flap repair | Subclavian flap repair had the lowest recurrence rate. Age influenced recurrence rates, with a high rate in the 1–5-year age group. |
| Uguz et al. (2010) [68] | 91 | 44 months | 12.1% | Extended end-to-end, End-to-end | Neonates had a higher recoarctation rate. Infants had a lower restenosis rate. |
| Jahangiri et al. (2000) [69] | 185 | 6.2 years | 6% | Subclavian flap | Subclavian flap angioplasty showed excellent long-term outcomes. Hypoplastic arch was not the primary site of recoarctation. |
| Burch et al. (2009) [70] | 167 | - | - | End-to-end | Suture type influenced outcomes in neonates and infants. |
| Sen et al. (2018) [71] | 75 | - | - | Balloon, Surgery | Balloon coarctoplasty had a higher reintervention rate. Age influenced the choice of surgical technique. |
| Rao et al. (1995) [72] | 29 | 4.5 years | - | Surgical, Balloon | Both surgical repair and balloon angioplasty had recoarctation cases. |
| Mohan et al. (2009) [73] | 60 | Short-term | - | Stent implantation | Stents effectively increased CoA diameter and reduced gradients in children. Weight did not significantly impact results. |
| Corno et al. (2001) [74] | 141 | 30 years | Varies | Various surgical | Recurrence rates varied by surgical approach, with no recoarctation in adults. |
| Padalino et al. (2019) [75] | 341 | 10.2 years | 4.5% | Extended end to end anastomosis, patch, or conduit interposition | Low recurrence rate, and recurrences managed with percutaneous procedures. |
| Kaushal et al. (2009) [76] | 201 | 5.0 years | 4% | Extended end-to-end anastomosis | stent implantation was successful. |
| Presbitero et al. (1987) [77] | 226 | 20 years | - | Various surgical | Late-onset hypertension was noted, and recurrence varied by the surgical approach. |
| Josep Rodes-Cabau et al. (2007) [78] | 80 | 3 years | Surgical Repair (77% End-to-End Anastomosis) | Surgical patients had higher complications and longer hospitalization. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, C.M.; Laforest, G.; Bulescu, C.; Jalal, Z.; Thambo, J.-B.; Iriart, X. From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions. J. Clin. Med. 2023, 12, 7350. https://doi.org/10.3390/jcm12237350
Vasile CM, Laforest G, Bulescu C, Jalal Z, Thambo J-B, Iriart X. From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions. Journal of Clinical Medicine. 2023; 12(23):7350. https://doi.org/10.3390/jcm12237350
Chicago/Turabian StyleVasile, Corina Maria, Gerald Laforest, Cristian Bulescu, Zakaria Jalal, Jean-Benoit Thambo, and Xavier Iriart. 2023. "From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions" Journal of Clinical Medicine 12, no. 23: 7350. https://doi.org/10.3390/jcm12237350
APA StyleVasile, C. M., Laforest, G., Bulescu, C., Jalal, Z., Thambo, J. -B., & Iriart, X. (2023). From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions. Journal of Clinical Medicine, 12(23), 7350. https://doi.org/10.3390/jcm12237350