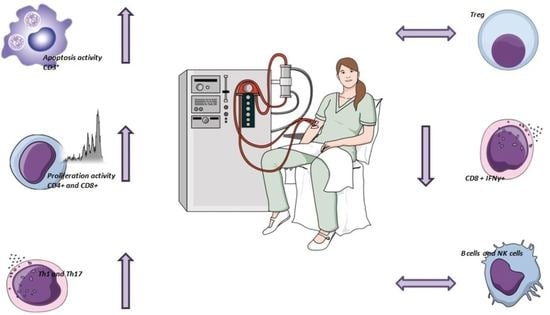
Impact of Single Hemodialysis Treatment on immune Cell Subpopulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Characteristics and Study Design
2.2. Hemodialysis Technique
2.3. Isolation of Peripheral Blood Mononucleate Cells
2.4. Flow Cytometry Analysis
2.5. Cell Proliferation Analysis
2.6. Annexin V with 7-Aminoactinomycin D (7-AAD) Assay
2.7. Statistical Analysis
3. Results
3.1. Demographic and Dialysis-Related Characteristics
3.2. Effects of HD Session on CD3+T-Cells and Apoptosis
3.3. Dialysis Session Increases the Proliferation Activity of CD4+ T and CD8+ Cells
3.4. Chronic Dialysis Treatment Increases the Percentage of Th17 and Th1 Cells and Decreases the reg/Th17 Ratio
3.5. Dialysis Session Reduces IFN-ꙋ Production by CD8 T Cells
3.6. Dialysis Does Not Alter B Cells Content but Decreases the NK
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.; Woo, K. Epidemiology of End-Stage Kidney Disease. Semin. Vasc. Surg. 2021, 34, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; Donati, G. The cardiovascular burden of end-stage renal disease patients. Minerva Urol. Nefrol. 2010, 62, 51–66. [Google Scholar] [PubMed]
- Xiong, J.; Qiao, Y.; Yu, Z.; Huang, Y.; Yang, K.; Ting, H.; Zhao, J. T-Lymphocyte Subsets Alteration, Infection and Renal Outcome in Advanced Chronic Kidney Disease. Front. Med. 2021, 8, 742419. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.B.; Stinghen, A.E. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit. Dial Int. 2008, 28, S183–S187. [Google Scholar] [CrossRef]
- Carrero, J.J.; Yilmaz, M.I. Cytokine Dysregulation in Chronic Kidney Disease: How Can We Treat It? Blood Purif. 2008, 26, 291–299. [Google Scholar] [CrossRef]
- Hakim, R.M. Clinical implications of hemodialysis membrane biocompatibility. Kidney Int. 1993, 44, 484–494. [Google Scholar] [CrossRef]
- Kara, I.H.; Yilmaz, M.E. The Evaluation of Immune Responses That Occur after HBV Infection and HBV Vaccination in Hemodialysis Patients. Vaccine 2004, 22, 3963–3967. [Google Scholar] [CrossRef]
- Jofré, R.; Rodriguez-Benitez, P. Inflammatory syndrome in patients on hemodialysis. J. Am. Soc. Nephrol. 2006, 17, S274–S280. [Google Scholar] [CrossRef]
- Poppelaars, F.; Faria, B. The Complement System in Dialysis: A Forgotten Story? Front. Immunol. 2018, 9, 71. [Google Scholar] [CrossRef]
- Melchior, P.; Erlenkötter, A. Complement Activation by Dialysis Membranes and Its Association with Secondary Membrane Formation and Surface Charge. Artif. Organs 2021, 45, 770–778. [Google Scholar] [CrossRef]
- Nilsson, B.; Ekdahl, K.N. The Role of Complement in Biomaterial-Induced Inflammation. Mol. Immunol. 2007, 44, 82–94. [Google Scholar] [CrossRef]
- Jasiulewicz, A.; Lisowska, K.A. Homeostatic “bystander” Proliferation of Human Peripheral Blood B Cells in Response to Polyclonal T-Cell Stimulation in Vitro. Int. Immunol. 2015, 27, 579–588. [Google Scholar] [CrossRef]
- Rysz, J.; Banach, M.; Cialkowska-Rysz, A.; Stolarek, R.; Barylski, M.; Drozdz, J.; Okonski, P. Blood Serum Levels of IL-2, IL-6, IL-8, TNF-Alpha and IL-1beta in Patients on Maintenance Hemodialysis. Cell. Mol. Immunol. 2006, 3, 151–154. [Google Scholar]
- Angeletti, A.; Zappulo, F. Immunological Effects of a Single Hemodialysis Treatment. Medicina 2020, 56, 71. [Google Scholar] [CrossRef]
- Moser, B.; Roth, G. Aberrant T Cell Activation and Heightened Apoptotic Turnover in End-Stage Renal Failure Patients: A Comparative Evaluation between Non-Dialysis, Haemodialysis, and Peritoneal Dialysis. Biochem. Biophys. Res. Commun. 2003, 308, 581–585. [Google Scholar] [CrossRef]
- Betjes, M.G.H.; Langerak, A.W. Premature Aging of Circulating T Cells in Patients with End-Stage Renal Disease. Kidney Int. 2011, 80, 208–817. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Debska-Slizien, A. Recombinant Human Erythropoietin Treatment of Chronic Renal Failure Patients Normalizes Altered Phenotype and Proliferation of CD4-Positive T Lymphocytes. Artif. Organs 2010, 34, E77–E84. [Google Scholar] [CrossRef]
- Xiang, F.F.; Zhu, J.M. Lymphocyte Depletion and Subset Alteration Correlate to Renal Function in Chronic Kidney Disease Patients. Ren. Fail. 2016, 38, 7–14. [Google Scholar] [CrossRef]
- Turner, J.E.; Paust, H.J. The Th17 Immune Response in Renal Inflammation. Kidney Int. 2012, 82, 72–83. [Google Scholar] [CrossRef]
- Barbi, J.; Pardoll, D. Treg Functional Stability and Its Responsiveness to the Microenvironment. Immunol. Rev. 2014, 259, 115–139. [Google Scholar] [CrossRef]
- Cravedi, P.; Manrique, J. Immunosuppressive effects of erythropoietin on human alloreactive T cells. J. Am. Soc. Nephrol. 2014, 25, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Abd Hamid, M.; Yao, X. Defective Interferon Gamma Production by Tumor-Specific CD8+ T Cells Is Associated With 5′Methylcytosine-Guanine Hypermethylation of Interferon Gamma Promoter. Front. Immunol. 2020, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, K.A.; Storoniak, H. T Cell Subpopulations and Cytokine Levels in Hemodialysis Patients. Hum. Immunol. 2022, 83, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK Cell Receptors. Annu. Rev. Immunol. 1998, 16, 359–393. [Google Scholar] [CrossRef]
- Gollapudi, P.; Yoon, J.W. Leukocyte Toll-like Receptor Expression in End-Stage Kidney Disease. Am. J. Nephrol. 2010, 31, 247–254. [Google Scholar] [CrossRef]
- Agrawal, S.; Gollapudi, P. Effects of End-Stage Renal Disease and Haemodialysis on Dendritic Cell Subsets and Basal and LPS-Stimulated Cytokine Production. Nephrol. Dial. Transplant. 2010, 25, 737–746. [Google Scholar] [CrossRef]
- Liszewski, M.K.; Kolev, M. Intracellular Complement Activation Sustains T Cell Homeostasis and Mediates Effector Differentiation. Immunity 2013, 39, 1143–1157. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Dębska-Ślizień, A. Hemodialysis Affects Phenotype and Proliferation of CD4-Positive T Lymphocytes. J. Clin. Immunol. 2012, 32, 189–200. [Google Scholar] [CrossRef]
- Dungey, M.; Hull, K.L. Inflammatory Factors and Exercise in Chronic Kidney Disease. Int. J. Endocrinol. 2013, 2013, 569831. [Google Scholar] [CrossRef]






| Characteristics | Filtryzer BGU1.6 (N = 12) | Theranova (N = 12) | HFR17 (N = 11) | p Value |
|---|---|---|---|---|
| Age (years) | 74 (54–88) | 74 (56–86) | 76.5 (55.2–87.3) | 0.989 |
| Women, n (%) | 6 (50) | 7 (58.3) | 6 (54.5) | 0.925 |
| BMI (Kg/m2) | 22.22 (19.87–28.11) | 27.9 (22.15–28) | 24.39 (23.18–32.5) | 0.74 |
| Neutrophils (109/L) | 4.3 (3.5–5.5) | 4.4 (3.6–7.3) | 4.3 (3.4–5.3) | 0.939 |
| Lymphocytes (109/L) | 1.2 (0.9–1.6) | 1.2 (0.9–1.5) | 1.18 (0.9–1.4) | 0.945 |
| Platelets (109/L) | 170 (105–227) | 168 (99.7–224.5) | 170 (101.5–229) | 0.983 |
| Ferritin (ng/mL) | 150 (60.7–272.5) | 149.5 (66–319) | 149.5 (63.2–248) | 0.952 |
| Transferrin saturation (mg/mL) | 25.7 (12.8–37.2) | 25.5 (13–37.7) | 25.5 (13.4–38) | 0.976 |
| Serum iron (µg/dL) | 41 (27.7–78) | 51 (30–78) | 47 (28.5–76.5) | 0.921 |
| Albumin (g/dL) | 3.6 (3.3–3.8) | 3.5 (3.4–4.2) | 3.5 (3.4–3.9) | 0.986 |
| NLI | 3.8 (2.8–5) | 3.6 (2.9–5) | 3.7 (2.9–4.87) | 0.983 |
| PLI | 134 (100–236.5) | 120.7 (89–233) | 134.3 (97.2–230.7) | 0.970 |
| CRP (mg/dL) | 0.35 (0.07–6.1) | 0.6 (0.21–0.8) | 0.15 (0.08–0.2) | 0.942 |
| Iron therapy (%) | 33.33 | 58.33 | 36.36 | / |
| EPO therapy (%) | 100 | 100 | 100 | / |
| Characteristics | Filtryzer BGU1.6 (N = 12) | Theranova (N = 12) | HFR17 (N = 11) | p Value |
|---|---|---|---|---|
| Time spent on dialysis (months) | 47 (19.5–60) | 44 (17.5–60) | 44.5 (15.5–60) | 0.903 |
| Type of dialysis (stHD/HDF) | 9/3 | 12/0 | 0/11 | / |
| Vascular access (AVF/CVC) | 6/6 | 5/7 | 8/3 | / |
| Blood flow (mL/min) | 300 (270–300) | 300 (275–300) | 300 (280–300) | 0.967 |
| Dialysis duration (min) | 240 (240–240) | 240 (240–240) | 240 (240–240) | 0.809 |
| Session per week | 3 | 3 | 3 | / |
| V men (L) | 17.8 (17–21.3) | 17.03 (15.1–18.9) | 18.6 (17.3–20.1) | 0.922 |
| V Women (L) | 13.3 (12.3–14.3) | 14.34 (13.3–16.1) | 17.8 (15.3–23.9) | 0.934 |
| Phosphorous (mg/dL) | 4.65 (3.85–6.38) | 5.1 (3.9–6.2) | 5.5 (4.35–6.08) | 0.810 |
| PTH | 305 (145.8–546.8) | 300 (116–473) | 314 (190–340) | 0.72 |
| Calcium (mg/dL) | 8.9 (8.08–9.5) | 8.3 (7.9–8.8) | 8.8 (8.73–9.22) | 0.881 |
| Delta on dry weight | 3.1 (2.07–4.05) | 2.2 (2–2.7) | 3.1 (2–3.5) | 0.69 |
| Characteristics | Filtryzer BGU1.6 (N = 12) | Theranova (N = 12) | HFR17 (N = 11) | p Value |
|---|---|---|---|---|
| Urea (mg/dL) | 141 (83–152.5) | 122 (83–165) | 112.5 (98.5–137.8) | 0.673 |
| B2M | 34.5 (27.8–33.4) | 29.9 (27.85–33.4) | 31.2 (25.3–42.6) | 0.864 |
| Potassium (mEq/L) | 4.9 (4.35–5.15) | 4.5 (4.3–5.7) | 5.95 (5.28–6.08) | 0.791 |
| Kappa chains (mg/L) | 144.6 (118.2–224.8) | 165.3 (110.1–211.6) | 215 (123–301.4) | 0.513 |
| Lamda chains (mg/L) | 127.2 (62.75–164.5) | 133.6 (94.8–138.3) | 121.3 (105.7–142) | 0.786 |
| Global kt/V | 1.58 (14.83–1.68) | 1.64 (1.52–1.67) | 1.66 (1.51–1.74) | 0.834 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadei, C.; Angeletti, A.; Pizzuti, V.; Zappulo, F.; Conte, D.; Cappuccilli, M.; Chiocchini, A.L.; Scrivo, A.; Apuzzo, D.; Mariggiò, M.A.; et al. Impact of Single Hemodialysis Treatment on immune Cell Subpopulations. J. Clin. Med. 2023, 12, 3107. https://doi.org/10.3390/jcm12093107
Donadei C, Angeletti A, Pizzuti V, Zappulo F, Conte D, Cappuccilli M, Chiocchini AL, Scrivo A, Apuzzo D, Mariggiò MA, et al. Impact of Single Hemodialysis Treatment on immune Cell Subpopulations. Journal of Clinical Medicine. 2023; 12(9):3107. https://doi.org/10.3390/jcm12093107
Chicago/Turabian StyleDonadei, Chiara, Andrea Angeletti, Valeria Pizzuti, Fulvia Zappulo, Diletta Conte, Maria Cappuccilli, Anna Laura Chiocchini, Anna Scrivo, Delia Apuzzo, Maria Addolorata Mariggiò, and et al. 2023. "Impact of Single Hemodialysis Treatment on immune Cell Subpopulations" Journal of Clinical Medicine 12, no. 9: 3107. https://doi.org/10.3390/jcm12093107
APA StyleDonadei, C., Angeletti, A., Pizzuti, V., Zappulo, F., Conte, D., Cappuccilli, M., Chiocchini, A. L., Scrivo, A., Apuzzo, D., Mariggiò, M. A., Gasperoni, L., Donati, G., & La Manna, G. (2023). Impact of Single Hemodialysis Treatment on immune Cell Subpopulations. Journal of Clinical Medicine, 12(9), 3107. https://doi.org/10.3390/jcm12093107