Effects of a Two-Month Exercise Training Program on Concurrent Non-Opiate Substance Use in Opioid-Dependent Patients during Substitution Treatment
Abstract
:1. Introduction
2. Materials and Methods
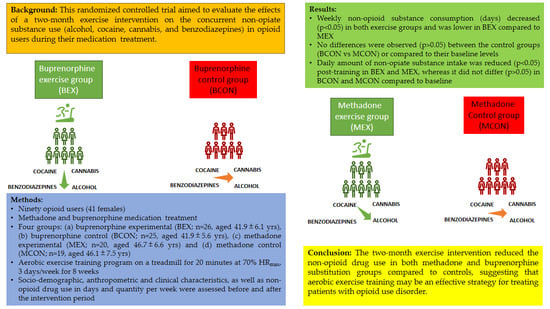
2.1. Design, Participants, Recruitment, and Experimental Procedures
2.1.1. Study Design
2.1.2. Exclusion Criteria and Sample Size
2.1.3. Experimental Procedures
2.1.4. Ethical Approval
2.2. Questionnaires, Patient history, and—Substance Use Measurements
2.3. Urine Drug Monitoring
Drop Test Method Implemented in OKANA’s Structures
2.4. Measurement of Substitute Dosage
2.5. Somatometric Measurements
2.6. Aerobic Exercise Training
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Medical History
3.3. Changes in BZD, Cannabis, and Cocaine Use
3.4. Changes in Alcohol Use
3.5. Changes in Dosage of Medication for Opioid Use Disorders
3.6. Changes in Health and Welfare
3.6.1. Days of Work and Education
3.6.2. Sports/Voluntary Days
3.6.3. Homelessness/Violence/Arrest Days
| Buprenorphine | p1 | Methadone | p1 | p2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Group | Control Group | Exercise Group | Control Group | ||||||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||||
| Days with sports/volunteering | |||||||||||
| 1st week | 1.58 (1.1) | 1.5 (1–3) | 1.4 (0.96) | 1 (1–2) | 0.556 | 0.7 (0.73) | 1 (0–1) | 0.63 (0.68) | 1 (0–1) | 0.793 | 0.007 |
| 2nd week | 1.65 (1.13) | 2 (1–3) | 2.84 (1.14) | 3 (2–3) | 0.001 | 0.85 (0.88) | 1 (0–1) | 1.79 (1.03) | 2 (1–2) | 0.004 | 0.016 |
| 3rd week | 1.65 (1.13) | 2 (1–3) | 2.32 (1.25) | 3 (1–3) | 0.050 | 0.85 (0.88) | 1 (0–1) | 1.42 (0.96) | 1 (1–2) | 0.057 | 0.016 |
| 4th week | 1.77 (1.24) | 2 (1–3) | 1.68 (1.03) | 2 (1–2) | 0.838 | 0.9 (0.91) | 1 (0–1.5) | 0.63 (0.83) | 0 (0–1) | 0.331 | 0.016 |
| 5th week | 2.04 (1.31) | 2 (1–3) | 1.4 (0.96) | 1 (1–2) | 0.066 | 1.05 (0.89) | 1 (0–2) | 0.53 (0.7) | 0 (0–1) | 0.053 | 0.009 |
| 6th week | 2.5 (1.39) | 3 (2–4) | 1.48 (0.96) | 1 (1–2) | 0.005 | 1.15 (0.93) | 1 (0–2) | 0.53 (0.7) | 0 (0–1) | 0.031 | 0.001 |
| 7th week | 2.92 (1.38) | 3 (2–4) | 1.32 (0.85) | 1 (1–2) | <0.001 | 1.6 (0.88) | 2 (1–2) | 0.53 (0.7) | 0 (0–1) | <0.001 | 0.001 |
| 8th week | 3.15 (1.35) | 3 (2–4) | 1.32 (0.85) | 1 (1–2) | <0.001 | 2 (0.86) | 2 (1–2.5) | 0.53 (0.7) | 0 (0–1) | <0.001 | 0.003 |
| 1th–8th week | 17.27 (9.66) | 19 (11–26) | 13.76 (7.46) | 12 (9–19) | 0.112 | 9.1 (6.56) | 9.5 (2–12.5) | 6.58 (5.78) | 4 (3–10) | 0.324 | 0.002 |
| Homeless/Violence/ Arrest | |||||||||||
| 1st–4th week | 1.96 (0.53) | 2 (2–2) | 2.08 (0.64) | 2 (2–2) | 0.462 | 4.2 (1.36) | 4 (3.5–5) | 4.21 (1.13) | 4 (4–5) | 0.988 | <0.001 |
| 5th–8th week | 1.35 (0.49) | 1 (1–2) | 2.08 (0.64) | 2 (2–2) | <0.001 | 3.1 (1.12) | 3 (2–4) | 4.11 (1.1) | 4 (4–5) | 0.010 | <0.001 |
| 1th–8th week | 3.31 (0.79) | 3 (3–4) | 4.16 (1.28) | 4 (4–4) | 0.005 | 7.3 (2.41) | 7 (6–9) | 8.32 (2.21) | 8 (8–10) | 0.202 | <0.001 |
| Physical and mental health/QoL | |||||||||||
| 1st week | 7 (1.13) | 7 (6–8) | 6.92 (0.95) | 7 (6–7) | 0.835 | 4.9 (1.12) | 5 (4–6) | 4.74 (1.37) | 5 (3–6) | 0.812 | <0.001 |
| 4th week | 13.12 (1.88) | 13.5 (12–14) | 11.2 (2.04) | 12 (9–12) | 0.001 | 11.1 (1.48) | 11 (10–12) | 7.47 (1.74) | 7 (6–10) | <0.001 | <0.001 |
| 8th week | 19.12 (2.08) | 19 (18–21) | 12.6 (2.53) | 13 (11–14) | <0.001 | 17.5 (1.73) | 17.5 (16.5–18) | 9.68 (2.14) | 9 (8–12) | <0.001 | 0.011 |
| 1th–8th week | 39.23 (4.47) | 41 (35–43) | 30.72 (5.06) | 32 (26–34) | <0.001 | 33.5 (3.65) | 33 (32–34) | 21.89 (4.81) | 21 (18–28) | <0.001 | <0.001 |
3.6.4. Quality of Life
4. Discussion
4.1. Alcohol
4.2. Cocaine
4.3. Cannabis
4.4. Benzodiazepines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenney, S.R.; Anderson, B.J.; Bailey, G.L.; Stein, M.D. Expectations about alcohol, cocaine, and benzodiazepine abstinence following inpatient heroin withdrawal management. Am. J. Addict. 2019, 28, 36–42. [Google Scholar] [CrossRef]
- Gottheil, E.; Sterling, R.C.; Weinstein, S.P. Diminished illicit drug use as a consequence of long-term methadone maintenance. J. Addict. Dis. 1993, 12, 45–57. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, P.A., Jr.; Sterling, R.; Weinstein, S.P. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. Am. J. Addict. 2000, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Church, S.H.; Rothenberg, J.L.; Sullivan, M.A.; Bornstein, G.; Nunes, E.V. Concurrent substance use and outcome in combined behavioral and naltrexone therapy for opiate dependence. Am. J. Drug Alcohol Abuse 2001, 27, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.; Klages, E.; Welzel, H.; Mann, K.; Croissant, B. Low efficacy of non-opioid drugs in opioid withdrawal symptoms. Addict. Biol. 2005, 10, 165–169. [Google Scholar] [CrossRef]
- Hassan, A.N.; Le Foll, B. Polydrug use disorders in individuals with opioid use disorder. Drug Alcohol Depend. 2019, 198, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, S.L.; Lunde, L.H.; Torsheim, T. Opioid and Polydrug Use Among Patients in Opioid Maintenance Treatment. Subst. Abuse Rehabil. 2020, 11, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Coffin, P.O.; Galea, S.; Ahern, J.; Leon, A.C.; Vlahov, D.; Tardiff, K. Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–1998. Addiction 2003, 98, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Kerr, T.; Fairbairn, N.; Tyndall, M.; Marsh, D.; Li, K.; Montaner, J.; Wood, E. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007, 87, 39–45. [Google Scholar] [CrossRef]
- Wang, L.; Min, J.E.; Krebs, E.; Evans, E.; Huang, D.; Liu, L.; Hser, Y.I.; Nosyk, B. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int. J. Drug Policy 2017, 49, 32–40. [Google Scholar] [CrossRef]
- Shah, N.G.; Galai, N.; Celentano, D.D.; Vlahov, D.; Strathdee, S.A. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988–2000. Drug Alcohol Depend. 2006, 83, 147–156. [Google Scholar] [CrossRef]
- Stenbacka, M.; Beck, O.; Leifman, A.; Romelsjo, A.; Helander, A. Problem drinking in relation to treatment outcome among opiate addicts in methadone maintenance treatment. Drug Alcohol Rev. 2007, 26, 55–63. [Google Scholar] [CrossRef]
- Mannelli, P.; Peindl, K.; Patkar, A.A.; Wu, L.T.; Tharwani, H.M.; Gorelick, D.A. Problem drinking and low-dose naltrexone-assisted opioid detoxification. J. Stud. Alcohol Drugs 2011, 72, 507–513. [Google Scholar] [CrossRef]
- Eiroa-Orosa, F.J.; Haasen, C.; Verthein, U.; Dilg, C.; Schafer, I.; Reimer, J. Benzodiazepine use among patients in heroin-assisted vs. methadone maintenance treatment: Findings of the German randomized controlled trial. Drug Alcohol Depend. 2010, 112, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Brands, B.; Blake, J.; Marsh, D.C.; Sproule, B.; Jeyapalan, R.; Li, S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J. Addict. Dis. 2008, 27, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Stockings, E.; Strang, J.; Marsden, J.; Hall, W.D. Illicit Drug Dependence. In Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, 3rd ed.; Patel, V., Chisholm, D., Dua, T., Laxminarayan, R., Medina-Mora, M.E., Eds.; American Psychiatric Publishing: Washington, DC, USA, 2016; Volume 4. [Google Scholar] [CrossRef]
- Williamson, A.; Darke, S.; Ross, J.; Teesson, M. The effect of persistence of cocaine use on 12-month outcomes for the treatment of heroin dependence. Drug Alcohol Depend. 2006, 81, 293–300. [Google Scholar] [CrossRef]
- EMCDDA. Polydrug Use: Health and Social Responses. Available online: https://www.emcdda.europa.eu/publications/mini-guides/polydrug-use-health-and-social-responses_en (accessed on 9 November 2021).
- Gili, A.; Lancia, M.; Mercurio, I.; Bacci, M.; Nicoletti, A.; Pelliccia, C.; Gambelunghe, C. Patterns of Prescription Medicine, Illicit Drugs, and Alcohol Misuse among High-Risk Population: A Factor Analysis to Delineate Profiles of Polydrug Users. Healthcare 2022, 10, 710. [Google Scholar] [CrossRef]
- Apantaku-Olajide, T.; Darker, C.D.; Smyth, B.P. Onset of cocaine use: Associated alcohol intoxication and psychosocial characteristics among adolescents in substance abuse treatment. J. Addict. Med. 2013, 7, 183–188. [Google Scholar] [CrossRef] [PubMed]
- McCance-Katz, E.F.; Kosten, T.R.; Jatlow, P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone--a multiple-dose study. Biol. Psychiatry 1998, 44, 250–259. [Google Scholar] [CrossRef]
- Emcdda. Spotlight on… Comorbid Substance Use and Mental Health Problems. Available online: https://www.emcdda.europa.eu/spotlights/comorbid-substance-use-and-mental-health-problems_en (accessed on 27 March 2022).
- Torres, E.; Hillman, A.R. Clinical Exercise Considerations for Opioid Addiction Recovery. J. Clin. Exerc. Physiol. 2021, 10, 117–125. [Google Scholar] [CrossRef]
- Mooney, L.J.; Rawson, R.A. Exercise for Substance Use Disorders. In Textbook of Addiction Treatment; Springer: Berlin/Heidelberg, Germany, 2021; pp. 493–503. [Google Scholar]
- Shreffler, J.; Genova, G.; Huecker, M. Physical activity and exercise interventions for individuals with opioid use disorder: A scoping review. J. Addict. Dis. 2022, 40, 452–462. [Google Scholar] [CrossRef]
- Abdullah, M.; Huang, L.C.; Lin, S.H.; Yang, Y.K. Dopaminergic and glutamatergic biomarkers disruption in addiction and regulation by exercise: A mini review. Biomarkers 2022, 27, 306–318. [Google Scholar] [CrossRef]
- Connor, J.P.; Gullo, M.J.; White, A.; Kelly, A.B. Polysubstance use: Diagnostic challenges, patterns of use and health. Curr. Opin. Psychiatry 2014, 27, 269–275. [Google Scholar] [CrossRef]
- Crummy, E.A.; O’Neal, T.J.; Baskin, B.M.; Ferguson, S.M. One Is Not Enough: Understanding and Modeling Polysubstance Use. Front. Neurosci. 2020, 14, 569. [Google Scholar] [CrossRef] [PubMed]
- Haukka, J.; Kriikku, P.; Mariottini, C.; Partonen, T.; Ojanpera, I. Non-medical use of psychoactive prescription drugs is associated with fatal poisoning. Addiction 2018, 113, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Alpers, S.E.; Furulund, E.; Pallesen, S.; Mamen, A.; Dyrstad, S.M.; Fadnes, L.T. The Role of Physical Activity in Opioid Substitution Therapy: A Systematic Review of Interventional and Observational Studies. Subst. Abuse 2022, 16, 11782218221111840. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, A.M.; Van Noppen, D.; Bailey, G.; Uebelacker, L.A.; Buman, M.; Stein, M.D. A Feasibility Study of a Peer-Facilitated Physical Activity Intervention in Methadone Maintenance. Ment. Health Phys. Act. 2021, 21, 100419. [Google Scholar] [CrossRef]
- Uebelacker, L.A.; Van Noppen, D.; Tremont, G.; Bailey, G.; Abrantes, A.; Stein, M. A pilot study assessing acceptability and feasibility of hatha yoga for chronic pain in people receiving opioid agonist therapy for opioid use disorder. J. Subst. Abuse Treat. 2019, 105, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Colledge, F.; Vogel, M.; Dursteler-Macfarland, K.; Strom, J.; Schoen, S.; Puhse, U.; Gerber, M. A pilot randomized trial of exercise as adjunct therapy in a heroin-assisted treatment setting. J. Subst. Abuse Treat. 2017, 76, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, F.; Camara-Sanchez, M.; Tremblay, J.F.; Riera-Rubio, V.J.; Gil-Paisan, L.; Lucia, A. Benefits of exercise training in Spanish prison inmates. Int. J. Sports Med. 2007, 28, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Chegol, R.; Gholami, M.; Matinhomaee, H.; Abednatanzi, H.; Ghazalian, F. The effect of aerobic and resistance training with different dose of Methadone on fibrinogen and lipid profile in addicted men. J. Sport Biosci. 2020, 12, 291–305. [Google Scholar]
- Ding, Z.; Ma, Z.; Yang, X.; Sun, Y. Effect of Eight-Month Exercise Intervention on Bone Outcomes of Young Opioid-Dependent Women. Int. J. Environ. Res. Public Health 2021, 18, 1336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Huang, X.D.; Hu, X.F.; Wang, S.Q.; Chen, K.; Wei, J.A.; Yan, L.; So, K.F.; Yuan, T.F.; Zhang, L. Physical exercise rescues cocaine-evoked synaptic deficits in motor cortex. Mol. Psychiatry 2021, 26, 6187–6197. [Google Scholar] [CrossRef]
- Brellenthin, A.G.; Koltyn, K.F. Exercise as an adjunctive treatment for cannabis use disorder. Am. J. Drug Alcohol Abuse 2016, 42, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gunillasdotter, V.; Andreasson, S.; Hallgren, M.; Jirwe, M. Exercise as treatment for alcohol use disorder: A qualitative study. Drug Alcohol Rev. 2022, 41, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Ashton, H. The treatment of benzodiazepine dependence. Addiction 1994, 89, 1535–1541. [Google Scholar] [CrossRef]
- Ellingsen, M.M.; Johannesen, S.L.; Martinsen, E.W.; Dahl, S.R.; Hallgren, M. Effects of Acute Exercise on Drug Craving, Self-Esteem, Mood, and Affect in Adults with Polysubstance Use Disorder: Protocol for a Multicenter Randomized Controlled Trial. JMIR Res. Protoc. 2020, 9, e18553. [Google Scholar] [CrossRef] [PubMed]
- Zschucke, E.; Heinz, A.; Strohle, A. Exercise and physical activity in the therapy of substance use disorders. Sci. World J. 2012, 2012, 901741. [Google Scholar] [CrossRef] [PubMed]
- Simonton, A.J.; Young, C.C.; Brown, R.A. Physical Activity Preferences and Attitudes of Individuals With Substance Use Disorders: A Review of the Literature. Issues Ment. Health Nurs. 2018, 39, 657–666. [Google Scholar] [CrossRef]
- Gimenez-Meseguer, J.; Tortosa-Martinez, J.; Cortell-Tormo, J.M. The Benefits of Physical Exercise on Mental Disorders and Quality of Life in Substance Use Disorders Patients. Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3680. [Google Scholar] [CrossRef]
- Jake-Schoffman, D.E.; Berry, M.S.; Donahue, M.L.; Christou, D.D.; Dallery, J.; Rung, J.M. Aerobic Exercise Interventions for Patients in Opioid Maintenance Treatment: A Systematic Review. Subst. Abuse 2020, 14, 1178221820918885. [Google Scholar] [CrossRef]
- Thompson, T.P.; Horrell, J.; Taylor, A.H.; Wanner, A.; Husk, K.; Wei, Y.; Creanor, S.; Kandiyali, R.; Neale, J.; Sinclair, J.; et al. Physical activity and the prevention, reduction, and treatment of alcohol and other drug use across the lifespan (The PHASE review): A systematic review. Ment. Health Phys. Act. 2020, 19, 100360. [Google Scholar] [CrossRef]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Weinstock, J.; Wadeson, H.K.; VanHeest, J.L. Exercise as an adjunct treatment for opiate agonist treatment: Review of the current research and implementation strategies. Subst. Abus. 2012, 33, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Viscoli, C.M.; Makuch, R.W.; Brass, L.M.; Horwitz, R.I. Stratified randomization for clinical trials. J. Clin. Epidemiol. 1999, 52, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Sobell, L.C.; Sobell, M.B.; Leo, G.I. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav. 2014, 28, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Raouf, M.; Bettinger, J.J.; Fudin, J. A Practical Guide to Urine Drug Monitoring. Fed. Pract. 2018, 35, 38–44. [Google Scholar] [PubMed]
- Beebe, L.H.; Smith, K.; Burk, R.; Dessieux, O.; Velligan, D.; Tavakoli, A.; Tennison, C. Effect of a motivational group intervention upon exercise self efficacy and outcome expectations for exercise in Schizophrenia Spectrum Disorders (SSDs). J. Am. Psychiatr. Nurses Assoc. 2010, 16, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R. An application of the Kolmogorov-Smirnov test for normality with estimated mean and variance. Psychol. Rep. 1968, 22, 570. [Google Scholar] [CrossRef]
- Seivewright, N. Community Treatment of Drug Misuse. More than Methadone; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Eder, H.; Fischer, G.; Gombas, W.; Jagsch, R.; Stühlinger, G.; Kasper, S. Comparison of Buprenorphine and Methadone Maintenance in Opiate Addicts. Eur. Addict. Res. 1998, 4 (Suppl. S1), 3–7. [Google Scholar] [CrossRef]
- RCGP. Guidancefor the Use of Buprenorphine for the Treatment of Opioid Depedence in Primary Care; Department of Health and Social Care; Royal College of General Practitioners: London, UK, 2004. [Google Scholar]
- Rudolph, K.E.; Williams, N.T.; Goodwin, A.T.S.; Shulman, M.; Fishman, M.; Diaz, I.; Luo, S.; Rotrosen, J.; Nunes, E.V. Buprenorphine & methadone dosing strategies to reduce risk of relapse in the treatment of opioid use disorder. Drug Alcohol Depend. 2022, 239, 109609. [Google Scholar] [CrossRef]
- Ramos-Matos, C.F.; Bistas, K.G.; Lopez-Ojeda, W. Fentanyl; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Blum, K.; Baron, D. Opioid Substitution Therapy: Achieving Harm Reduction While Searching for a Prophylactic Solution. Curr. Pharm. Biotechnol. 2019, 20, 180–182. [Google Scholar] [CrossRef]
- Fenn, J.M.; Laurent, J.S.; Sigmon, S.C. Increases in body mass index following initiation of methadone treatment. J. Subst. Abuse Treat. 2015, 51, 59–63. [Google Scholar] [CrossRef]
- Schlienz, N.J.; Huhn, A.S.; Speed, T.J.; Sweeney, M.M.; Antoine, D.G. Double jeopardy: A review of weight gain and weight management strategies for psychotropic medication prescribing during methadone maintenance treatment. Int. Rev. Psychiatry 2018, 30, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Puhl, R.M.; Heuer, C.A. Obesity stigma: Important considerations for public health. Am. J. Public Health 2010, 100, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Rozanova, J.; Marcus, R.; Taxman, F.S.; Bojko, M.J.; Madden, L.; Farnum, S.O.; Mazhnaya, A.; Dvoriak, S.; Altice, F.L. Why People Who Inject Drugs Voluntarily Transition Off Methadone in Ukraine. Qual. Health Res. 2017, 27, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Elman, I.; Howard, M.; Borodovsky, J.T.; Mysels, D.; Rott, D.; Borsook, D.; Albanese, M. Metabolic and Addiction Indices in Patients on Opioid Agonist Medication-Assisted Treatment: A Comparison of Buprenorphine and Methadone. Sci. Rep. 2020, 10, 5617. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Semahegn, A.; Torpey, K.; Manu, A.; Assefa, N.; Tesfaye, G.; Ankomah, A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Langleben, D.D.; Lynch, K.G.; Wang, G.J.; Elman, I.; Wiers, C.E.; Shi, Z. Association between body mass index and treatment completion in extended-release naltrexone-treated patients with opioid dependence. Front. Psychiatry 2023, 14, 1247961. [Google Scholar] [CrossRef]
- Han, B.H.; Cotton, B.P.; Polydorou, S.; Sherman, S.E.; Ferris, R.; Arcila-Mesa, M.; Qian, Y.; McNeely, J. Geriatric Conditions Among Middle-aged and Older Adults on Methadone Maintenance Treatment: A Pilot Study. J. Addict. Med. 2022, 16, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Caviness, C.M.; Bird, J.L.; Anderson, B.J.; Abrantes, A.M.; Stein, M.D. Minimum recommended physical activity, and perceived barriers and benefits of exercise in methadone maintained persons. J. Subst. Abuse Treat. 2013, 44, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.M.R.; Drucker, A.M.; Alikhan, A.; Bercovitch, L.; Cohen, D.E.; Darr, J.M.; Eichenfield, L.F.; Frazer-Green, L.; Paller, A.S.; Silverberg, J.I.; et al. American Academy of Dermatology Guidelines: Awareness of comorbidities associated with atopic dermatitis in adults. J. Am. Acad. Dermatol. 2022, 86, 1335–1336.e18. [Google Scholar] [CrossRef] [PubMed]
- Paparrigopoulos, T.; Dalla, C.; Pillarisetti, A. The addictions; Crete University Press: Heraklion, Greece, 2018. [Google Scholar]
- Weinstock, J.; Alessi, S.M.; Petry, N.M. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend. 2007, 87, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Arsuffi, L.; Scarborough, N. A service evaluation of the Behavioural Treatment for Substance Abuse (BTSA) programme for forensic dual diagnosis populations. J. Forensic Psychol. Res. Pract. 2023, 23, 201–226. [Google Scholar] [CrossRef]
- Ye, X.; Liu, R. Intervention Effect of Aerobic Exercise on Physical Fitness, Emotional State and Mental Health of Drug Addicts: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2272. [Google Scholar] [CrossRef]
- Cabrera, I. Role of exercise in managing substance use disorders. Prescriber 2020, 31, 15–19. [Google Scholar] [CrossRef]
- Gimenez-Meseguer, J.; Tortosa-Martinez, J.; de los Remedios Fernandez-Valenciano, M. Benefits of Exercise for the Quality of Life of Drug-Dependent Patients. J. Psychoactive Drugs 2015, 47, 409–416. [Google Scholar] [CrossRef]
- Neale, J.; Nettleton, S.; Pickering, L. Heroin users’ views and experiences of physical activity, sport and exercise. Int. J. Drug Policy 2012, 23, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Nikraftar, N.S.; Feyzi, Y.F.; Ramzani, F.; Nikbakht-Zadeh, M.; Amini, M.; Arezoomandan, M.; Shiehmorteza, M.; Arezoomandan, R. Comparison of psychological symptoms and cognitive functions in patients under maintenance treatment with methadone or buprenorphine, current opioid users and healthy subjects. Asian J. Psychiatry 2021, 58, 102603. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, R.; Nash, M.; Peperzak, K.A.; Ziga, T.M.; Dinges, E.M.; Delgado, C.; Wu, J.; Terman, G.W.; Dale, R.C.D. Comparison Between Preoperative Methadone and Buprenorphine Use on Postoperative Opioid Requirement. Clin. J. Pain 2022, 38, 311–319. [Google Scholar] [CrossRef]
- Yarborough, B.J.H.; Stumbo, S.P.; McCarty, D.; Mertens, J.; Weisner, C.; Green, C.A. Methadone, buprenorphine and preferences for opioid agonist treatment: A qualitative analysis. Drug Alcohol Depend. 2016, 160, 112–118. [Google Scholar] [CrossRef]
- Obekpa, E.O.; McCurdy, S.A.; Schick, V.; Markham, C.M.; Gallardo, K.R.; Wilkerson, J.M. Health-related quality of life and recovery capital among recovery residents taking medication for opioid use disorder in Texas. Front. Public Health 2023, 11, 1284192. [Google Scholar] [CrossRef]
- El-Bassel, N.; Gilbert, L.; Hunt, T.; Wu, E.; Oga, E.A.; Mukherjee, T.I.; Campbell, A.N.C.; Sabounchi, N.; Gutnick, D.; Kerner, R.; et al. Using community engagement to implement evidence-based practices for opioid use disorder: A data-driven paradigm & systems science approach. Drug Alcohol Depend. 2021, 222, 108675. [Google Scholar] [CrossRef]
- Taylor, S.E. Concurrent Polydrug Use Behaviors among Opioid Using Emerging Adults: Perceived Life Satisfaction and Influencers. Ph.D. Thesis, Indiana University, Fort Wayne, IN, USA, 2020. [Google Scholar]
- Bobashev, G.; Tebbe, K.; Peiper, N.; Hoffer, L. Polydrug use among heroin users in Cleveland, OH. Drug Alcohol Depend. 2018, 192, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chu, I.H.; Lin, Y.J.; Wu, W.L.; Chang, Y.K.; Lin, I.M. Effects of Yoga on Heart Rate Variability and Mood in Women: A Randomized Controlled Trial. J. Altern. Complement Med. 2015, 21, 789–795. [Google Scholar] [CrossRef]
- Linke, S.E.; Gallo, L.C.; Norman, G.J. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Ann. Behav. Med. 2011, 42, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, T.; Chen, J.; Lu, Y.; Zhou, C.; Chang, Y.K. Acute Aerobic Exercise Ameliorates Cravings and Inhibitory Control in Heroin Addicts: Evidence From Event-Related Potentials and Frequency Bands. Front. Psychol. 2020, 11, 561590. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Galea, L.A.; Nagamatsu, L.S.; Erickson, K.I.; Liu-Ambrose, T. Personalising exercise recommendations for brain health: Considerations and future directions. Br. J. Sports Med. 2017, 51, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, M.; Vancampfort, D.; Giesen, E.S.; Lundin, A.; Stubbs, B. Exercise as treatment for alcohol use disorders: Systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1058–1064. [Google Scholar] [CrossRef]
- Weinstock, J.; Farney, M.R.; Elrod, N.M.; Henderson, C.E.; Weiss, E.P. Exercise as an Adjunctive Treatment for Substance Use Disorders: Rationale and Intervention Description. J. Subst. Abuse Treat. 2017, 72, 40–47. [Google Scholar] [CrossRef]
- Simonton, A.J.; Young, C.C.; Johnson, K.E. Physical Activity Interventions to Decrease Substance Use in Youth: A Review of the Literature. Subst. Use Misuse 2018, 53, 2052–2068. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, K.; Li, N.; Hurr, C.; Luo, J. The Relationship between Different Amounts of Physical Exercise, Internal Inhibition, and Drug Craving in Individuals with Substance-Use Disorders. Int. J. Environ. Res. Public Health 2021, 18, 2436. [Google Scholar] [CrossRef]
- Linke, S.E.; Ussher, M. Exercise-based treatments for substance use disorders: Evidence, theory, and practicality. Am. J. Drug Alcohol Abus. 2015, 41, 7–15. [Google Scholar] [CrossRef]
- Moriarty, T.; Bourbeau, K.; Zuhl, M. Exercise and Neural Adaptations: Designing a Novel Treatment for Alcohol Addiction. Altern. Ther. Health Med. 2020, 26, 48–57. [Google Scholar]
- Psarianos, A.; Chryssanthopoulos, C.; Paparrigopoulos, T.; Philippou, A. The Role of Physical Exercise in Opioid Substitution Therapy: Mechanisms of Sequential Effects. Int. J. Mol. Sci. 2023, 24, 4763. [Google Scholar] [CrossRef]
- EMCDDA. Polydrug Use: Health and Social Responses; Health and Social Responses; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2020. [Google Scholar]
- Melguizo-Ibanez, E.; Zurita-Ortega, F.; Gonzalez-Valero, G.; Puertas-Molero, P.; Badicu, G.; Greco, G.; Cataldi, S.; Fischetti, F. Alcohol, Tobacco and Cannabis Consumption on Physical Activity and Physical and Social Self-Concept in Secondary School Students: An Explanatory Model Regarding Gender. Int. J. Environ. Res. Public Health 2022, 19, 243. [Google Scholar] [CrossRef]
- Abernathy, K.; Chandler, L.J.; Woodward, J.J. Alcohol and the prefrontal cortex. Int. Rev. Neurobiol. 2010, 91, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, L.; Hardy, L.; Mathew, A.R.; Hitsman, B. Negative mood-induced alcohol-seeking is greater in young adults who report depression symptoms, drinking to cope, and subjective reactivity. Exp. Clin. Psychopharmacol. 2018, 26, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2013, 35, 155–173. [Google Scholar] [PubMed]
- Glass, J.M.; Buu, A.; Adams, K.M.; Nigg, J.T.; Puttler, L.I.; Jester, J.M.; Zucker, R.A. Effects of alcoholism severity and smoking on executive neurocognitive function. Addiction 2009, 104, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Abrantes, A.M.; Minami, H.; Read, J.P.; Marcus, B.H.; Jakicic, J.M.; Strong, D.R.; Dubreuil, M.E.; Gordon, A.A.; Ramsey, S.E.; et al. A preliminary, randomized trial of aerobic exercise for alcohol dependence. J. Subst. Abuse Treat. 2014, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Georgakouli, K.; Manthou, E.; Georgoulias, P.; Ziaka, A.; Fatouros, I.G.; Mastorakos, G.; Koutedakis, Y.; Theodorakis, Y.; Jamurtas, A.Z. Exercise training reduces alcohol consumption but does not affect HPA-axis activity in heavy drinkers. Physiol. Behav. 2017, 179, 276–283. [Google Scholar] [CrossRef]
- Karoly, H.C.; Stevens, C.J.; Thayer, R.E.; Magnan, R.E.; Bryan, A.D.; Hutchison, K.E. Aerobic exercise moderates the effect of heavy alcohol consumption on white matter damage. Alcohol Clin. Exp. Res. 2013, 37, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Manthou, E.; Georgakouli, K.; Fatouros, I.G.; Gianoulakis, C.; Theodorakis, Y.; Jamurtas, A.Z. Role of exercise in the treatment of alcohol use disorders. Biomed. Rep. 2016, 4, 535–545. [Google Scholar] [CrossRef]
- De La Garza, R., 2nd; Yoon, J.H.; Thompson-Lake, D.G.; Haile, C.N.; Eisenhofer, J.D.; Newton, T.F.; Mahoney, J.J., 3rd. Treadmill exercise improves fitness and reduces craving and use of cocaine in individuals with concurrent cocaine and tobacco-use disorder. Psychiatry Res. 2016, 245, 133–140. [Google Scholar] [CrossRef]
- Sinha, R.; Fuse, T.; Aubin, L.R.; O’Malley, S.S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 2000, 152, 140–148. [Google Scholar] [CrossRef]
- Lynch, W.J.; Piehl, K.B.; Acosta, G.; Peterson, A.B.; Hemby, S.E. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol. Psychiatry 2010, 68, 774–777. [Google Scholar] [CrossRef]
- Rupp, T.; Perrey, S. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur. J. Appl. Physiol. 2008, 102, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Pasic, J.; Zarkowski, P.; Nordstrom, K.; Wilson, M.P. Psychiatric Emergencies for Clinicians: Emergency Department Management of Cocaine-Related Presentations. J. Emerg. Med. 2017, 53, 383–387. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. Recent Changes in Europe’s Cocaine Market: Results from an EMCDDA Trendspotter Study; EMCDDA Trendspotter Study; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2022. [Google Scholar]
- Robison, L.S.; Alessi, L.; Thanos, P.K. Chronic forced exercise inhibits stress-induced reinstatement of cocaine conditioned place preference. Behav. Brain Res. 2018, 353, 176–184. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef]
- Zlebnik, N.E.; Carroll, M.E. Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacology 2015, 232, 3507–3513. [Google Scholar] [CrossRef]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Abrantes, A.M.; Read, J.P.; Marcus, B.H.; Jakicic, J.; Strong, D.R.; Oakley, J.R.; Ramsey, S.E.; Kahler, C.W.; Stuart, G.G.; et al. A Pilot Study of Aerobic Exercise as an Adjunctive Treatment for Drug Dependence. Ment. Health Phys. Act. 2010, 3, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mustafaoglu, R.; Gorek Dilektasli, A.; Demir, R.; Zirek, E.; Birinci, T.; Kaya Mutlu, E.; Evren, C.; Razak Ozdincler, A. Exercise capacity, lung and respiratory muscle function in substance use disorders. Pulmonology 2022, in press. [Google Scholar] [CrossRef]
- Rahimimoghadam, Z.; Rahemi, Z.; Mirbagher Ajorpaz, N.; Sadat, Z. Effects of Pilates exercise on general health of hemodialysis patients. J. Bodyw. Mov. Ther. 2017, 21, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Boon, M.; van Dorp, E.; Broens, S.; Overdyk, F. Combining opioids and benzodiazepines: Effects on mortality and severe adverse respiratory events. Ann. Palliat. Med. 2020, 9, 542–557. [Google Scholar] [CrossRef]
- EMCDDA. Non-Medical Use of Medicines: Health and Social Responses; Health and Social Responses; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2021. [Google Scholar]
- Novak, S.P.; Hakansson, A.; Martinez-Raga, J.; Reimer, J.; Krotki, K.; Varughese, S. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 2016, 16, 274. [Google Scholar] [CrossRef]
- Hernandez, I.; He, M.; Brooks, M.M.; Zhang, Y. Exposure-Response Association Between Concurrent Opioid and Benzodiazepine Use and Risk of Opioid-Related Overdose in Medicare Part D Beneficiaries. JAMA Netw. Open 2018, 1, e180919. [Google Scholar] [CrossRef] [PubMed]
- Higgitt, A.C.; Lader, M.H.; Fonagy, P. Clinical management of benzodiazepine dependence. Br. Med. J. (Clin. Res. Ed.) 1985, 291, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Ernstsen, L.; Zotcheva, E.; Sui, X.; Engstrom, M.; Martinez-Velilla, N.; Bjerkeset, O.; Bjorvatn, B.; Havnen, A. Association Between Cardiorespiratory Fitness and Incident Purchase of Hypnotic Drugs in Adults: The HUNT Study. Mayo Clin. Proc. 2023, 98, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wang, Y.; Li, R.; Zhou, C. Impact of physical exercise on substance use disorders: A meta-analysis. PLoS ONE 2014, 9, e110728. [Google Scholar] [CrossRef]
- Dremencov, E.; Csatlosova, K.; Durisova, B.; Moravcikova, L.; Lacinova, L.; Jezova, D. Effect of Physical Exercise and Acute Escitalopram on the Excitability of Brain Monoamine Neurons: In Vivo Electrophysiological Study in Rats. Int. J. Neuropsychopharmacol. 2017, 20, 585–592. [Google Scholar] [CrossRef]


| Treatment Outcome Profile HTOP | Data Type |
|---|---|
| History of Drug Use: | |
| Quantitative |
| Quantitative |
| Multi-selection |
| Quantitative |
| Quantitative |
| Quantitative |
| Quantitative |
| Health and Welfare | |
| Single-selection |
| Single-selection |
| Single-selection |
| Quantitative |
| Single-selection |
| Buprenorphine | p1 | Methadone | p1 | p2 | |||
|---|---|---|---|---|---|---|---|
| Exercise Group | Control Group | Exercise Group | Control Group | ||||
| Ν = 26; 28.9% | Ν = 25; 27.8% | Ν = 20; 22.2% | Ν = 19; 21.1% | ||||
| Ν (%) | Ν (%) | Ν (%) | Ν (%) | ||||
| Age (years), mean (SD) | 41.9 (6.1) | 41.9 (5.6) | 0.983 + | 46.7 (6.6) | 46.1 (7.5) | 0.794 + | 0.014 + |
| Gender | |||||||
| Men | 15 (57.7) | 11 (44.0) | 0.328 ++ | 12 (60.0) | 11 (57.9) | 0.894 ++ | 0.875 ++ |
| Women | 11 (42.3) | 14 (56.0) | 8 (40.0) | 8 (42.1) | |||
| BMI (kg/m2), mean (SD) | 23 (2.4) | 22.9 (2.3) | 0.911 + | 25 (3.4) | 25.3 (1.8) | 0.742 + | 0.019+ |
| BMI (kg/m2) | |||||||
| Normal | 22 (84.6) | 16 (64.0) | 0.091 ++ | 9 (45.0) | 8 (42.1) | 0.855 ++ | 0.004 ++ |
| Overweight/Obese | 4 (15.4) | 9 (36.0) | 11 (55.0) | 11 (57.9) | |||
| Age at first use, mean (SD) | 21 (2.9) | 22.2 (3.3) | 0.175 + | 17.5 (2.5) | 18.2 (3.2) | 0.449 + | <0.001 + |
| Diagnosis | |||||||
| F.19 | 5 (19.2) | 2 (8.0) | - | 10 (50.0) | 7 (36.8) | - | - |
| F.19–F.30 | 0 (0.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) | |||
| F.19–F.32 | 0 (0.0) | 0 (0.0) | 1 (5.0) | 2 (10.5) | |||
| F.19–F.40 | 1 (3.8) | 0 (0.0) | 3 (15.0) | 2 (10.5) | |||
| F.19–F.41.1 | 2 (7.7) | 2 (8.0) | 0 (0.0) | 0 (0.0) | |||
| F.20–7.29 | 0 (0.0) | 0 (0.0) | 1 (5.0) | 2 (10.5) | |||
| F.30–F.39 | 0 (0.0) | 0 (0.0) | 1 (5.0) | 2 (10.5) | |||
| F.40–F.48 | 5 (19.2) | 10 (40.0) | 3 (15.0) | 4 (21.1) | |||
| f.41 | 5 (19.2) | 2 (8.0) | 0 (0.0) | 0 (0.0) | |||
| F.41 | 7 (26.9) | 7 (28.0) | 0 (0.0) | 0 (0.0) | |||
| F.41–F.39 | 1 (3.8) | 2 (8.0) | 0 (0.0) | 0 (0.0) | |||
| HIV | 3 (11.5) | 0 (0.0) | 0.080 ++ | 6 (30.0) | 2 (10.5) | 0.235 ‡‡ | 0.149 ‡‡ |
| Hepatitis | 3 (11.5) | 4 (16.0) | 0.703 ‡‡ | 4 (20.0) | 0 (0.0) | 0.106 ‡‡ | 0.682 ‡‡ |
| Number of relapses during rehabilitation. Mean (SD) | 1 (1–2) | 1 (1–2) | 0.802 ‡ | 4 (3–4) | 4 (3–4) | 0.767 ‡ | <0.001 ‡ |
| Buprenorphine | p1 | Methadone | p1 | p2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Group | Control Group | Exercise Group | Control Group | ||||||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||||
| Cannabis use | |||||||||||
| Total number of days | 9.77 (11.08) | 0 (0–20) | 12.32 (10.68) | 14 (0–21) | 0.384 | 14.65 (11.1) | 18.5 (0–24) | 19.37 (11.09) | 21 (13–27) | 0.245 | 0.110 |
| Mean daily quantity (1st–4th week) | 1.31 (1.49) | 0 (0–3) | 1.68 (1.52) | 2 (0–2) | 0.560 | 2.3 (1.69) | 3 (0–4) | 2.63 (1.38) | 3 (2–4) | 0.683 | 0.033 |
| Mean daily quantity (5th–8th week) | 0.65 (0.8) | 0 (0–1) | 1.6 (1.35) | 2 (0–2) | 0.010 | 1.5 (1.28) | 2 (0–3) | 2.53 (1.31) | 3 (2–3) | 0.016 | 0.022 |
| Benzodiazepine use | |||||||||||
| Total number of days | 25.23 (16.89) | 32 (0–40) | 27.84 (19.38) | 37 (0–42) | 0.336 | 34.85 (15.46) | 39.5 (26–44.5) | 36.84 (18.53) | 45 (24–50) | 0.311 | 0.031 |
| Mean daily quantity (1st–4th week) | 1.58 (1.03) | 2 (0–2) | 1.52 (1) | 2 (0–2) | 0.800 | 2.25 (0.91) | 2 (2–3) | 2.11 (1.05) | 2 (2–3) | 0.735 | 0.012 |
| Mean daily quantity (5th–8th week) | 0.77 (0.51) | 1 (0–1) | 1.44 (1) | 2 (0–2) | 0.005 | 1.45 (0.69) | 2 (1–2) | 2 (1.11) | 2 (1–3) | 0.035 | 0.001 |
| Cocaine | |||||||||||
| Total number of days | 1.08 (2.48) | 0 (0–0) | 2.96 (5.41) | 0 (0–0) | 0.424 | 3.95 (5.77) | 0 (0–9.5) | 8.26 (7.38) | 12 (0–14) | 0.037 | 0.105 |
| Buprenorphine | p1 | Methadone | p1 | p2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Group | Control Group | Exercise Group | Control Group | ||||||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||||
| Days consuming at least 5 drinks | |||||||||||
| 1st week | 2.62 (1.63) | 3 (2–4) | 2.8 (1.58) | 3 (2–4) | 0.869 | 4.05 (1.67) | 4 (3–5) | 4.21 (1.44) | 4 (3–5) | 0.772 | 0.007 |
| 2nd week | 2.54 (1.58) | 3 (2–4) | 2.16 (1.21) | 2 (2–3) | 0.168 | 4 (1.59) | 4 (3–5) | 3 (1.2) | 3 (2–4) | 0.023 | 0.003 |
| 3rd week | 2.42 (1.47) | 3 (2–3) | 2 (1.15) | 2 (2–3) | 0.095 | 3.55 (1.73) | 4 (2.5–5) | 3 (1.45) | 3 (2–4) | 0.188 | 0.022 |
| 4th week | 2.19 (1.36) | 3 (2–3) | 2.56 (1.45) | 3 (2–3) | 0.432 | 3.1 (1.52) | 3 (2–4) | 3.89 (1.49) | 4 (3–5) | 0.211 | 0.046 |
| 5th week | 2 (1.3) | 2 (1–3) | 2.8 (1.47) | 3 (2–4) | 0.022 | 2.65 (1.42) | 3 (2–4) | 3.89 (1.33) | 4 (3–5) | 0.015 | 0.121 |
| 6th week | 1.73 (1.34) | 2 (0–3) | 2.72 (1.43) | 3 (2–3) | 0.008 | 2.6 (1.6) | 3 (1.5–4) | 4 (1.53) | 4 (3–5) | 0.019 | 0.056 |
| 7th week | 1.5 (1.36) | 2 (0–2) | 2.64 (1.5) | 3 (2–4) | 0.007 | 2.3 (1.53) | 2.5 (1.5–3) | 4.32 (1.45) | 5 (3–5) | <0.001 | 0.069 |
| 8th week | 1.42 (1.36) | 1.5 (0–2) | 2.64 (1.5) | 3 (2–4) | 0.005 | 2.15 (1.6) | 2 (0.5–3) | 4.21 (1.58) | 4 (3–5) | 0.001 | 0.120 |
| Total number of days consuming at least 5 drinks | 16.42 (10.42) | 19 (13–24) | 20.32 (10.95) | 22 (16–27) | 0.155 | 24.4 (11.38) | 25.5 (17–34.5) | 30.53 (11.09) | 30 (24–38) | 0.143 | 0.024 |
| Mean daily quantity of alcohol (1st- 4th week) | 2.88 (1.8) | 3 (3–4) | 3.32 (1.31) | 3 (3–4) | 0.588 | 4.3 (1.66) | 4 (3–5.5) | 4.89 (1.29) | 5 (4–6) | 0.244 | 0.011 |
| Mean daily quantity of alcohol (5th- 8th week) | 1.58 (1.17) | 2 (1–2) | 3.24 (1.16) | 3 (3–4) | <0.001 | 2.75 (1.45) | 3 (2–3.5) | 4.79 (1.23) | 5 (4–5) | <0.001 | 0.004 |
| Buprenorphine | p1 | Methadone | p1 | p2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Group | Control Group | Exercise Group | Control Group | ||||||||
| Substitute Dosage (mg/Week) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||
| Program onset | 10.38 (1.5) | 10 (10–12) | 10 (1.41) | 10 (10–10) | 0.333 | 88.5 (21.59) | 90 (70–105) | 84.21 (18.05) | 90 (60–100) | 0.627 | <0.001 |
| 1st week | 10.38 (1.5) | 10 (10–12) | 10 (1.41) | 10 (10–10) | 0.333 | 88.25 (21.23) | 90 (70–105) | 84.21 (18.05) | 90 (60–100) | 0.627 | <0.001 |
| 2nd week | 10.38 (1.5) | 10 (10–12) | 10 (1.41) | 10 (10–10) | 0.333 | 88.25 (21.23) | 90 (70–105) | 83.68 (17.63) | 90 (60–95) | 0.587 | <0.001 |
| 3rd week | 10.23 (1.53) | 10 (10–12) | 9.76 (1.56) | 10 (8–10) | 0.278 | 87 (20.74) | 87.5 (70–102.5) | 83.68 (17.63) | 90 (60–95) | 0.732 | <0.001 |
| 4th week | 9.46 (1.56) | 10 (8–10) | 9.76 (1.56) | 10 (8–10) | 0.564 | 85 (20.84) | 85 (67.5–102.5) | 83.68 (17.63) | 90 (60–95) | 1.000 | <0.001 |
| 5th week | 9.38 (1.68) | 10 (8–10) | 9.76 (1.56) | 10 (8–10) | 0.506 | 82 (20.99) | 82.5 (65–97.5) | 84.21 (18.05) | 90 (60–100) | 0.590 | <0.001 |
| 6th week | 9 (1.72) | 9 (8–10) | 9.76 (1.56) | 10 (8–10) | 0.138 | 79.25 (20.79) | 80 (62.5–95) | 83.68 (18.84) | 90 (60–100) | 0.453 | <0.001 |
| 7th week | 8.31 (1.76) | 8 (8–10) | 9.6 (1.41) | 10 (8–10) | 0.007 | 75.75 (21.54) | 75 (60–92.5) | 82.11 (19.03) | 85 (60–100) | 0.257 | <0.001 |
| 8th week | 7.92 (1.92) | 8 (6–8) | 8.96 (1.31) | 8 (8–10) | 0.022 | 73.75 (20.96) | 70 (60–90) | 81.58 (18.86) | 85 (60–100) | 0.198 | <0.001 |
| Total substitute dosage (mg) | 75.08 (12.04) | 75 (66–84) | 77.6 (11.25) | 78 (68–80) | 0.636 | 665.53 (167.61) | 665 (525–830) | 666.84 (145.27) | 710 (480–785) | 0.918 | <0.001 |
| Buprenorphine | p1 | Methadone | p1 | p2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Group | Control Group | Exercise Group | Control Group | ||||||||
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | ||||
| Working days | |||||||||||
| Week 1 | 2.5 (2.18) | 2 (0–5) | 2.24 (1.96) | 2 (0–4) | 0.693 | 1.45 (1.79) | 0 (0–3) | 1.37 (1.8) | 0 (0–3) | 0.864 | 0.080 |
| Week 2 | 2.5 (2.18) | 2 (0–5) | 2.24 (1.96) | 2 (0–4) | 0.693 | 1.45 (1.79) | 0 (0–3) | 1.37 (1.8) | 0 (0–3) | 0.864 | 0.080 |
| Week 3 | 2.5 (2.18) | 2 (0–5) | 2.24 (1.96) | 2 (0–4) | 0.693 | 1.45 (1.79) | 0 (0–3) | 1.37 (1.8) | 0 (0–3) | 0.864 | 0.080 |
| Week 4 | 2.77 (2.25) | 4 (0–5) | 2.24 (1.96) | 2 (0–4) | 0.426 | 1.7 (2.03) | 0 (0–4) | 1.37 (1.8) | 0 (0–3) | 0.608 | 0.068 |
| Week 5 | 3.42 (1.9) | 4 (2–5) | 2.56 (1.89) | 2 (2–4) | 0.110 | 2.25 (2) | 3 (0–4) | 1.68 (1.8) | 2 (0–3) | 0.286 | 0.025 |
| Week 6 | 3.46 (1.92) | 4 (2–5) | 2.56 (1.89) | 2 (2–4) | 0.090 | 2.3 (2.05) | 3 (0–4) | 1.68 (1.8) | 2 (0–3) | 0.273 | 0.034 |
| Week 7 | 3.5 (1.92) | 4 (2–5) | 2.56 (1.89) | 2 (2–4) | 0.082 | 2.45 (2.11) | 3.5 (0–4) | 1.68 (1.8) | 2 (0–3) | 0.164 | 0.043 |
| Week 8 | 3.5 (1.92) | 4 (2–5) | 2.72 (1.74) | 2 (2–4) | 0.109 | 2.5 (2.14) | 4 (0–4) | 1.68 (1.8) | 2 (0–3) | 0.139 | 0.050 |
| Total days of working | 24.15 (15.66) | 26 (8–40) | 19.36 (14.58) | 16 (16–32) | 0.263 | 15.55 (14.84) | 14.5 (0–26) | 12.21 (13.89) | 12 (0–24) | 0.378 | 0.056 |
| Buprenorphine | p1 | Methadone | p1 | p2 | |||||||
| Exercise Group | Control Group | Exercise Group | Control Group | ||||||||
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | ||||
| Education Days | |||||||||||
| Week 1 | 1.65 (1.74) | 2 (0–2) | 1.56 (1.66) | 1 (0–2) | 0.905 | 1.05 (1.5) | 0 (0–2) | 1.05 (1.18) | 0 (0–2) | 0.727 | 0.211 |
| Week 2 | 1.65 (1.74) | 2 (0–2) | 1.48 (1.66) | 1 (0–2) | 0.752 | 1.15 (1.5) | 0 (0–2) | 1.05 (1.18) | 0 (0–2) | 0.937 | 0.321 |
| Week 3 | 1.77 (1.86) | 2 (0–2) | 1.56 (1.66) | 1 (0–2) | 0.781 | 1.2 (1.54) | 0 (0–2) | 1.05 (1.18) | 0 (0–2) | 0.950 | 0.320 |
| Week 4 | 1.81 (1.92) | 2 (0–2) | 1.56 (1.66) | 1 (0–2) | 0.751 | 1.25 (1.62) | 0 (0–2) | 1.05 (1.18) | 0 (0–2) | 0.925 | 0.314 |
| Week 5 | 2.08 (1.98) | 2 (0–4) | 1.88 (1.72) | 2 (0–3) | 0.785 | 1.55 (1.88) | 1 (0–2) | 1.05 (1.18) | 0 (0–2) | 0.600 | 0.360 |
| Week 6 | 2.31 (2.02) | 2 (0–4) | 1.88 (1.72) | 2 (0–3) | 0.461 | 1.55 (1.88) | 1 (0–2) | 1.05 (1.18) | 0 (0–2) | 0.600 | 0.196 |
| Week 6 | 2.38 (2.04) | 2 (0–4) | 1.88 (1.72) | 2 (0–3) | 0.383 | 1.6 (1.9) | 1 (0–2.5) | 1.05 (1.18) | 0 (0–2) | 0.509 | 0.197 |
| Week 8 | 2.38 (2.04) | 2 (0–4) | 1.88 (1.72) | 2 (0–3) | 0.383 | 1.65 (1.93) | 1 (0–3) | 1.05 (1.18) | 0 (0–2) | 0.426 | 0.228 |
| Total days of education | 16.04 (14.7) | 16 (0–24) | 13.68 (13.1) | 8 (0–23) | 0.610 | 11 (13.3) | 4 (0–17.5) | 8.42 (9.42) | 0 (0–16) | 0.626 | 0.227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psarianos, A.; Chryssanthopoulos, C.; Theocharis, A.; Paparrigopoulos, T.; Philippou, A. Effects of a Two-Month Exercise Training Program on Concurrent Non-Opiate Substance Use in Opioid-Dependent Patients during Substitution Treatment. J. Clin. Med. 2024, 13, 941. https://doi.org/10.3390/jcm13040941
Psarianos A, Chryssanthopoulos C, Theocharis A, Paparrigopoulos T, Philippou A. Effects of a Two-Month Exercise Training Program on Concurrent Non-Opiate Substance Use in Opioid-Dependent Patients during Substitution Treatment. Journal of Clinical Medicine. 2024; 13(4):941. https://doi.org/10.3390/jcm13040941
Chicago/Turabian StylePsarianos, Alexandros, Costas Chryssanthopoulos, Athanasios Theocharis, Thomas Paparrigopoulos, and Anastassios Philippou. 2024. "Effects of a Two-Month Exercise Training Program on Concurrent Non-Opiate Substance Use in Opioid-Dependent Patients during Substitution Treatment" Journal of Clinical Medicine 13, no. 4: 941. https://doi.org/10.3390/jcm13040941
APA StylePsarianos, A., Chryssanthopoulos, C., Theocharis, A., Paparrigopoulos, T., & Philippou, A. (2024). Effects of a Two-Month Exercise Training Program on Concurrent Non-Opiate Substance Use in Opioid-Dependent Patients during Substitution Treatment. Journal of Clinical Medicine, 13(4), 941. https://doi.org/10.3390/jcm13040941