Evaluation of the Value of Histological Examination for the Prediction of Genetic Thoracic Proximal Aortopathies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patient Recruitment
2.3. Histological Evaluation of Aortic Wall Specimens
2.4. Genetic Testing
2.5. Scoring System for the Clinical Assessment of Cardiovascular and Histopathological Findings
- →
- Arterial hypertension = 1 point;
- →
- Hyperlipoproteinemia = 1 point;
- →
- Current or past nicotine abuse = 1 point;
- →
- Diabetes mellitus = 1 point.
- →
- Presence of media degeneration = 1 point;
- →
- Changes in the extracellular matrix = 1 point;
- →
- Changes in elastic fibers = 1 point;
- →
- Changes in smooth muscle cells = 1 point;
- →
- Changes in collagen tissue = 1 point;
- →
- Summarized assessment by the pathologist regarding whether the findings may correspond to a genetic connective tissue disorder = 1 point.
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
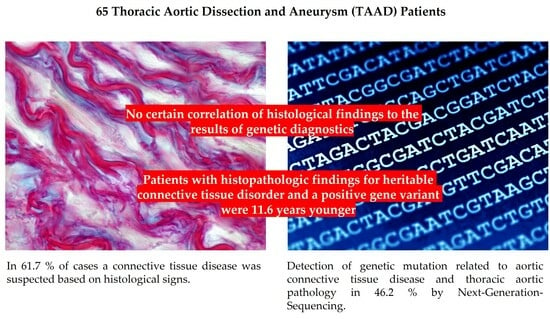
3.2. Histologic Findings
3.3. Genetic Results
3.4. Correlation between Histological and Human Genetic Findings
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumann, F.; Makaloski, V.; Diehm, N. Aortenaneurysma und dissektion: Epidemiologie, Pathophysiologie und Diagnostik. Der Internist 2013, 54, 535–542. [Google Scholar] [CrossRef]
- Melo, R.G.; Gonçalo Silva, D.; Lopes, A.; Alves, M.; Caldeira, D.; Fernandes, R.; Mendes Pedro, L. Incidence and Prevalence of Thoracic Aortic Aneurysms: A Systematic Review and Meta-analysis of Population-Based Studies. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 1–16. [Google Scholar] [CrossRef]
- Bossone, E.; Eagle, K.A. Epidemiology and Management of Aortic Disease: Aortic Aneurysms and Acute Aortic Syndromes. Nat. Rev. Cardiol. 2020, 18, 331–348. [Google Scholar] [CrossRef]
- Gudbjartsson, T.; Ahlsson, A.; Geirsson, A.; Gunn, J.; Hjortdal, V.; Jeppsson, A.; Mennander, A.; Zindovic, I.; Olsson, C. Acute Type A Aortic Dissection—A Review. Scand. Cardiovasc. J. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Yuan, X.; Mitsis, A.; Nienaber, C.A. Current Understanding of Aortic Dissection. Life 2022, 12, 1606. [Google Scholar] [CrossRef]
- Gao, J.; Wu, H.; Shi, X.; Huo, Z.; Zhang, J.; Liang, Z. Comparison of Next-Generation Sequencing, Quantitative PCR, and Sanger Sequencing for Mutation Profiling of EGFR, KRAS, PIK3CA and BRAF in Clinical Lung Tumors. Clin. Lab. 2016, 62, 689–696. [Google Scholar] [CrossRef]
- Jing, C.; Mao, X.; Wang, Z.; Sun, K.; Ma, R.; Wu, J.; Cao, H. Next-generation sequencing-based detection of EGFR, KRAS, BRAF, NRAS, PIK3CA, Her-2 and TP53 mutations in patients with non-small cell lung cancer. Mol. Med. Rep. 2018, 18, 2191–2197. [Google Scholar] [CrossRef]
- Garcia, J.; Kamps-Hughes, N.; Geiguer, F.; Couraud, S.; Sarver, B.; Payen, L.; Ionescu-Zanetti, C. Sensitivity, Specificity, and Accuracy of a Liquid Biopsy Approach Utilizing Molecular Amplification Pools. Sci. Rep. 2021, 11, 10761. [Google Scholar] [CrossRef] [PubMed]
- Halushka, M.; Angelini, A.; Bartoloni, G.; Basso, C.; Batoroeva, L.; Bruneval, P.; Buja, L.M. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pa-thology: II. Noninflammatory degenerative diseases—Nomenclature and diagnostic criteria. Cardiovasc. Pathol. 2016, 25, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Vinholo, T.F.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Nonsyndromic Thoracic Aortic Aneurysms and Dissections—Is Screening Possible? Semin. Thorac. Cardiovasc. Surg. 2019, 31, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V.; et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; A Zafar, M.; Liu, Y.; Chen, J.F.; Li, Y.; A Ziganshin, B.; Ellauzi, H.; Mukherjee, S.K.; A Rizzo, J.; A Elefteriades, J. Fate of the unoperated ascending thoracic aortic aneurysm: Three-decade experience from the Aortic Institute at Yale University. Eur. Heart J. 2023, 44, 4579–4588. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: Document Covering Acute and Chronic Aortic Diseases of the Thoracic and Abdominal Aorta of the Adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; A Elefteriades, J.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardiothorac. Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef]
- Grewal, N.; Velders, B.J.J.; Gittenberger-de Groot, A.C.; Poelmann, R.; Klautz, R.J.M.; Van Brakel, T.J.; Lindeman, J.H.N. A Systematic Histopathologic Evaluation of Type-A Aortic Dissections Implies a Uniform Multiple-Hit Causation. J. Cardiovasc. Dev. Dis. 2021, 8, 12. [Google Scholar] [CrossRef]
- Osada, H.; Kyogoku, M.; Matsuo, T.; Kanemitsu, N. Histopathological evaluation of aortic dissection: A comparison of congenital versus acquired aortic wall weakness. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 277–283. [Google Scholar] [CrossRef]
- Surman, T.L.; Abrahams, J.M.; Manavis, J.; Finnie, J.; O’rourke, D.; Reynolds, K.J.; Edwards, J.; Worthington, M.G.; Beltrame, J. Histological regional analysis of the aortic root and thoracic ascending aorta: A complete analysis of aneurysms from root to arch. J. Cardiothorac. Surg. 2021, 16, 255. [Google Scholar] [CrossRef]
- Stejskal, V.; Karalko, M.; Smolak, P.; Hanusova, M.; Steiner, I. Medial degeneration and atherosclerosis show discrete variance around the circumference of ascending aorta aneurysms. Virchows Arch. 2022, 481, 731–738. [Google Scholar] [CrossRef]
- Waters, K.M.; Rooper, L.M.; Guajardo, A.; Halushka, M.K. Histopathologic differences partially distinguish syndromic aortic diseases. Cardiovasc. Pathol. 2017, 30, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Vinholo, T.F.; Brownstein, A.J.; Ziganshin, B.A.; Zafar, M.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: 2019 Update and Clinical Implications. Aorta 2019, 7, 99–107. [Google Scholar] [CrossRef]
- Poninska, J.K.; Bilinska, Z.T.; Franaszczyk, M.; Michalak, E.; Rydzanicz, M.; Szpakowski, E.; Pollak, A.; Milanowska, B.; Truszkowska, G.; Chmielewski, P.; et al. Next-generation sequencing for diagnosis of thoracic aortic aneurysms and dissections: Diagnostic yield, novel mutations and genotype phenotype correlations. J. Transl. Med. 2016, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Mahlmann, A.; Elzanaty, N.; Saleh, M.; Irqsusi, M.; Rastan, A.; Leip, J.L.; Behrendt, C.-A.; Ghazy, T. Prevalence of Genetic Variants and Deep Phenotyping in Patients with Thoracic Aortic Aneurysm and Dissection: A Cross-Sectional Single-Centre Cohort Study. J. Clin. Med. 2024, 13, 461. [Google Scholar] [CrossRef] [PubMed]



| Diagnostic Criteria | Microscopic Histological Assessment |
|---|---|
| Overall media degeneration | Grading: none, mild, moderate, severe |
| Changes in the extracellular matrix | Mucoid extracellular matrix accumulation (MEMA) |
| Changes in elastic fibers | Elastic fiber fragmentation and/or loss (EFF) Elastic fiber thinning Elastic fiber disorganization (EFD) |
| Changes in smooth muscle cells | Smooth muscle cell nucleus loss (SMCL) Smooth muscle cell disorganization Laminar medial collapse (LMC) |
| Changes in collagen tissue | Medial fibrosis |
| Presence of atherosclerosis | Grading: mild, moderate, severe |
| Summary assessment by the pathologist as to whether the findings may correspond to a genetic connective tissue disorder | Concordant, nonconcordant |
| Gene name | Protein | Associated (Aortic) Disease/Syndrome |
|---|---|---|
| ACTA2 (NM_001613.2) | Smooth muscle α-actin | TAAD (thoracic aortic aneurysm and dissection) |
| AEBP1 (NM_001129.4) | AE binding protein 1 | Abdominal aortic aneurysm (AAA) Ehlers–Danlos syndrome (EDS) |
| BGN (NM_01711.5) | Biglycan | TAAD Meester–Loeys syndrome |
| COL1A1 (NM_000088.3) | Collagen 1 α1 chain | TAAD EDS |
| COL3A1 (NM_000090.3) | Collagen 3 α1 chain | TAAD EDS, vascular Type (IV) |
| COL4A5 (NM_000495.3) | Collagen 4 α5 chain | TAAD Defect collagen Type IV, Alport syndrome |
| COL5A1 (NM_000093.4) | Collagen 5 α1 chain | TAA (thoracic aortic aneurysm) EDS, classical Type I |
| COL5A2 (NM_000393.4) | Collagen 5 α2 chain | TAA EDS, classical Type II |
| EFEMP2 (FBLN4) (NM_016938.4) | Pin-4 | TAA, other arterial aneurysms Skin laxation, (autosomal recessive) Type Ib |
| ELN (NM_000501.3, NM_001278939.1) | Elastin | TAAD Loose skin (autosomal dominant) |
| FBLN5 (NM_006329.3) | Pin 5 | TAAD Cutis laxa, macular degeneration |
| FBN1 (NM_000138.4) | Fibrillin-1 | TAAD, AAA, and other arterial aneurysms Marfan syndrome |
| FBN2 (NM_001999.3) | Fibrillin-2 | TAAD |
| FLNA (NM_001110556.2) | Filamin A | TAAD |
| FOXE3 (NM_012186.2) | Forkhead box | TAAD |
| GATA5 (NM_080473.4) | GATA binding protein | TAA |
| LOX (NM_002317.6) | Lysyl oxidase | AAA/dissection |
| MAT2A (NM_005911.5) | Methionine adenosyl -transferase II α | TAA |
| MFAP5 (NM_003480.3) | Microfibril-associated glycoprotein 2 | TAAD |
| MYH11 (NM_002474.2) | Smooth muscle myosin heavy chain | TAAD |
| MYLK (NM_053025.3) | Myosin light chain kinase | TAAD |
| NOTCH1 (NM_017617.4) | Notch receptor 1 | TAAD |
| NOTCH3 | Notch receptor 3 | TAAD |
| PLOD1 (NM_000302.3) | Procollagen-lysine,2-oxoglutarate 5-dioxygenase 1 | TAAD EDS |
| PRKG1 (NM_006258.3) | Type I cGMP-dependent protein kinase | TAAD, AAA |
| SKI (NM_003036.3) | Sloan Kettering proto-oncoprotein | TAA Shprintzen–Goldberg syndrome |
| SLC2A10 (NM_030777.3) | Glucose transporter 10 | TAA, other arterial aneurysms Arterial torsion syndrome |
| SMAD3 (NM_005902.3) | SMAD3 | TAAD, AAA, other arterial aneurysms Loeys–Dietz syndrome Type III |
| SMAD4 (NM_005359.5) | SMAD4 | TAAD |
| SMAD6 (NM_005585.4) | SMAD6 | TAA |
| TAB2 (NM_015093.5) | TGF-beta activated kinase 1 (MAP3K7) binding protein 2 | TAAD |
| TGFB2 (NM_003238.4) | TGF-β2 | TAAD Loeys–Dietz syndrome Type IV |
| TGFB3 (NM_003239.4) | TGF-β3 | TAAD, AAA/dissection, other arterial aneurysms Loeys–Dietz syndrome Type V |
| TGFBR1 (NM_004612.2) | TGF-β receptor Type I | TAAD, AAA, other arterial aneurysms Loeys–Dietz syndrome Type I |
| TGFBR2 (NM_003242.5) | TGF- β receptor type II | TAAD, AAA, other arterial aneurysms Loeys–Dietz syndrome type II |
| Class 1 | Non-disease-causing/non-pathogenic or clinically irrelevant |
| Class 2 | Likely non-disease-causing/non-pathogenic or clinically irrelevant |
| Class 3 | Unclassified variant |
| Class 4 | Likely disease-causing and pathogenic |
| Class 5 | Disease-causing/pathogenic |
| Patient Characteristics | Men | Women | p-Value | |
|---|---|---|---|---|
| Number n (%) | 65 (100) | 50 (76.9) | 15 (23.1) | |
| Age (in years) | 48.4 ± 10.6 | 48.1 ± 10.3 | 49.6 ± 12.1 | n.s., p = 0.635 |
| TAD n (%) | 36 (55.4)
| 28 (77.8) | 8 (22.2) | n.s., p = 0.830 |
| TAA n (%) | 29 (44.6) | 22 (75.9) | 7 (24.1) | |
| Performed surgical procedures in TAD In all cases, treatment of acute disease | Only ascending aortic replacement: 2 (5.6) Bentall/David technique: 16 (44.4) + Hemiarch replacement: 12 (33.3) Frozen elephant trunk: 6 (16.7) | |||
| Performed surgical procedures in TAA Acute disease: 11 (37.9) Chronic disease: 18 (62.1) | Root remodeling: 1 (3.5) Bentall/David technique: 17 (58.6) + Hemiarch replacement: 6 (20.7) Aortic arch replacement: 5 (17.2) | |||
| Bicuspid aortic valve | 7 (10.8) | |||
Aortic regurgitation
| 12 of the total 65 patients (18.5) 4 of 7 patients (57.1) | |||
| Cardiovascular risk factors | n (in %) | |||
| Arterial hypertension | 52 (80.0) | |||
| Former or current smoking status | 19 (29.2) | |||
| Hyperlipidemia | 10 (15.4) | |||
| Diabetes mellitus | 4 (6.2) | |||
| Comorbidities | ||||
| Coronary heart disease | 8 (12.3) | |||
| Carotid stenosis/stroke | 3 (4.6) | |||
| Peripheral arterial disease | 1 (1.5) | |||
| Chronic renal insufficiency | 5 (7.7) | |||
| COPD | 1 (1.5) | |||
| Diagnostic Criteria | n (%) | |
|---|---|---|
| All histological signs | Summary assessment by the pathologist as to whether the findings may correspond to a connective tissue disorder | 29 (61.7) |
| Overall media degeneration | Without differentiation | 38 (80.9) |
| Changes in elastic fibers | Total | 38 (80.9) |
| Elastic fiber fragmentation and/or loss (EFF) | 30 (63.8) | |
| Elastic fiber thinning | 3 (6.4) | |
| Elastic fiber disorganization (EFD) | 5 (10.6) | |
| Changes in the extracellular matrix | Mucoid extracellular matrix accumulation (MEMA) | 31 (66.0) |
| Grading | Mild | 8 (17.0) |
| Moderate | 8 (17.0) | |
| Severe | 15 (31.9) | |
| Presence of atherosclerosis | Total | 24 (51.1) |
| Grading | Mild | 17 (36.2) |
| Moderate | 3 (6.4) | |
| Severe | 4 (8.5) | |
| Changes in smooth muscle cells | Smooth muscle cell nucleus loss (SMCL) Laminar medial collapse (LMC) Smooth muscle cell disorganization | 3 (6.4) |
| Changes in collagen tissue | Without differentiation | 1 (2.1) |
| n (%) | Men n (%) | Women n (%) | p-Value | |
|---|---|---|---|---|
| Patients | 65 (100) | 50 (76.9) | 15 (23.1) | n.s., p = 0.525 |
| Detected mutation | 30 (46.2) | 22 (73.3) | 8 (26.7) | |
| No mutation detected | 35 | 28 (80.0) | 7 (20.0) | |
| Mean age (in years) | ||||
| Total cohort | 48.4 ± 10.6 | n.s., p = 0.039 | ||
| Patients with mutation | 46.0 ± 11.8 | |||
| Patients without mutation | 50.5 ± 9.2 | |||
| Name of the Gene | Number of Mutation Detections n (%) | Classic Mutation n (%) | Mutation Variants n (%) |
|---|---|---|---|
| FBN1 | 10 (33.3) | 2 (6.7) | 8 (26.7) |
| MYH11 | 6 (20.0) | 6 (20.0) | |
| TGFB2 | 3 (10.0) | 3 (10.0) | |
| MYLK | 2 (6.7) | 2 (6.7) | |
| NOTCH1 | 2 (6.7) | 2 (6.7) | |
| TGFBR1 | 2 (6.7) | 2 (6.7) | |
| ACTA2 | 1 (3.3) | 1 (3.3) | |
| COL3A1 | 1 (3.3) | 1 (3.3) | |
| NOTCH3 | 1 (3.3) | 1 (3.3) | |
| PRKG1 | 1 (3.3) | 1 (3.3) | |
| SMAD3 | 1 (3.3) | 1 (3.3) | |
| SMAD6 | 1 (3.3) | 1 (3.3) | |
| TGFBR2 | 1 (3.3) | 1 (3.3) | |
| 45, X | 1 (3.3) | 1 (3.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlmann, A.; Rodionov, R.N.; Behrendt, C.-A.; Leip, J.L.; Lackner, H.K.; Eraqi, M.; Elzanaty, N.; Ghazy, T. Evaluation of the Value of Histological Examination for the Prediction of Genetic Thoracic Proximal Aortopathies. J. Clin. Med. 2024, 13, 1838. https://doi.org/10.3390/jcm13071838
Mahlmann A, Rodionov RN, Behrendt C-A, Leip JL, Lackner HK, Eraqi M, Elzanaty N, Ghazy T. Evaluation of the Value of Histological Examination for the Prediction of Genetic Thoracic Proximal Aortopathies. Journal of Clinical Medicine. 2024; 13(7):1838. https://doi.org/10.3390/jcm13071838
Chicago/Turabian StyleMahlmann, Adrian, Roman N. Rodionov, Christian-Alexander Behrendt, Jennifer Lynne Leip, Helmut Karl Lackner, Mohamed Eraqi, Nesma Elzanaty, and Tamer Ghazy. 2024. "Evaluation of the Value of Histological Examination for the Prediction of Genetic Thoracic Proximal Aortopathies" Journal of Clinical Medicine 13, no. 7: 1838. https://doi.org/10.3390/jcm13071838
APA StyleMahlmann, A., Rodionov, R. N., Behrendt, C. -A., Leip, J. L., Lackner, H. K., Eraqi, M., Elzanaty, N., & Ghazy, T. (2024). Evaluation of the Value of Histological Examination for the Prediction of Genetic Thoracic Proximal Aortopathies. Journal of Clinical Medicine, 13(7), 1838. https://doi.org/10.3390/jcm13071838