Alterations in Corneal Sensitivity, Staining and Biomechanics of Alopecia Areata Patients: Novel Findings in a Case-Control Study
Abstract
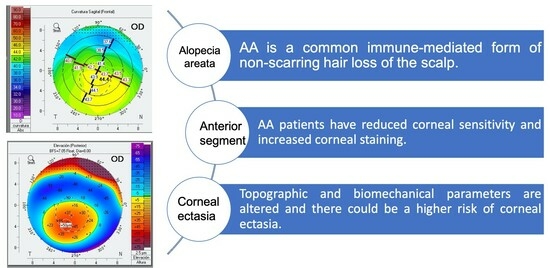
:1. Introduction
2. Methods Section
2.1. Design of the Study
2.2. Participants and Recruitment
2.3. Outcomes and Assessments
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safavi, K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch. Dermatol. 1992, 128, 702. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Etzioni, A.; Paus, R. Alopecia areata. N. Engl. J. Med. 2012, 366, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Sicco, K.L.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, A.; Alsantali, A.; Wang, E.; McElwee, K.J.; Shapiro, J. Alopecia areata update. Part I. Clinical picture, histopathology, and pathogenesis. J. Am. Acad. Dermatol. 2010, 62, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Hordinsky, M.K.; Price, V.H.; Roberts, J.L.; Shapiro, J.; Canfield, D.; Duvic, M.; King, L.E.; McMichael, A.J.; Randall, V.A.; et al. Alopecia areata investigational assessment guidelines—Part II. National Alopecia Areata Foundation. J. Am. Acad. Dermatol. 2004, 51, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Orecchia, G.; Bianchi, P.; Malvezzi, F.; Stringa, M.; Mele, F.; Douville, H. Lens changes in alopecia areata. Dermatologica 1988, 176, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Colombati, S.; Caponeri, G.; Ciliberti, C.; Tosti, G.; Bosi, M.; Veronesei, S. Ocular abnormalities occurring with alopecia areata. Dermatologica 1985, 170, 69–73. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.A.; Giavedoni, P.; Keller, J.; Sainz-de-la-Maza, M.T.; Ferrando, J. Ocular findings in patients with alopecia areata: Role of ultra-wide-field retinal imaging. Immunol. Res. 2014, 60, 356–360. [Google Scholar] [CrossRef]
- Pandhi, D.; Singal, A.; Gupta, R.; Das, G. Ocular alterations in patients of alopecia areata. J. Dermatol. 2009, 36, 262–268. [Google Scholar] [CrossRef]
- Camacho, F.; Tosti, A.; Randall, V.; Price, V. Tricología: Enfermedades Del Folículo Pilosebáceo. In Manifestaciones Oculares En La Alopecia Areata; Aula Médica Ediciones: Madrid, Spain, 2013; pp. 779–782. [Google Scholar]
- Nemet, A.Y.; Vinker, S.; Bahar, I.; Kaiserman, I. The association of keratoconus with immune disorders. Cornea. 2010, 29, 1261–1264. [Google Scholar] [CrossRef]
- Bassiouny, R.M.; Awad, E.A.; Gaafar, W.; Kyrillos, F.A.; Samra, W.A.A.; Abdelhameed, A.G. Corneal Tomographic Analysis Among Patients With Thyroid Gland Dysfunction. J. Refract. Surg. 2021, 37, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Amador, C.; Tormanen, K.; Ghiam, S.; Saghizadeh, M.; Arumugaswami, V.; Kumar, A.; Kramerov, A.A.; Ljubimov, A.V. Systemic diseases and the cornea. Exp. Eye Res. 2021, 204, 108455. [Google Scholar] [CrossRef] [PubMed]
- Esmer, O.; Karadag, R.; Cakici, O.; Bilgili, S.G.; Demircan, Y.T.; Bayramlar, H.; Karadag, A.S. Ocular findings in patients with alopecia areata. Int. J. Dermatol. 2016, 55, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.A.; Brunsting, L.A. Cataracts in alopecia areata. Report of five cases. Arch. Dermatol. 1963, 88, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Ergin, C.; Acar, M.; Akıs, H.K. Ocular findings in alopecia areata. Int. J. Dermatol. 2015, 54, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Recupero, S.M.; Abdolrahimzadeh, S.; De Dominicis, M.; Mollo, R.; Carboni, I.; Rota, L.; Calvieri, S. Ocular alterations in alopecia areata. Eye 1999, 13, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Prie, B.; Voiculescu, V.; Ionescu-Bozdog, O.; Petrutescu, B.; Iosif, L.; Gaman, L.; Clatici, V.; Stoian, I.; Giurcaneanu, C. Oxidative stress and alopecia areata. J. Med. Life 2015, 8, 43–46. [Google Scholar] [PubMed]
- Oltulu, P.; Oltulu, R.; Turk, H.B.; Turk, N.; Kilinc, F.; Belviranli, S.; Mirza, E.; Ataseven, A. The ocular surface findings in alopecia areata patients: Clinical parameters and impression cytology. Int. Ophthalmol. 2022, 42, 7–12. [Google Scholar] [CrossRef]
- Rahman, E.Z.; Lam, P.K.; Chu, C.K.; Moore, Q.; Pflugfelder, S.C. Corneal Sensitivity in Tear Dysfunction and its Correlation With Clinical Parameters and Blink Rate. Am. J. Ophthalmol. 2015, 160, 858–866.e5. [Google Scholar] [CrossRef]
- Koçak Altintas, A.G.; Gül, Ü.; Duman, S. Bilateral keratoconus associated with Hashimoto’s disease, alopecia areata and atopic keratoconjunctivitis. Eur. J. Ophthalmol. 1999, 9, 130–133. [Google Scholar] [CrossRef]
- Elham, R.; Jafarzadehpur, E.; Hashemi, H.; Amanzadeh, K.; Shokrollahzadeh, F.; Yekta, A.; Khabazkhoob, M. Keratoconus diagnosis using Corvis ST measured biomechanical parameters. J. Curr. Ophthalmol. 2017, 29, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Augustin, V.A.; Son, H.S.; Baur, I.; Zhao, L.; Auffarth, G.U.; Khoramnia, R. Detecting subclinical keratoconus by biomechanical analysis in tomographically regular keratoconus fellow eyes. Eur. J. Ophthalmol. 2021, 32, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Akcam, H.T.; Karagun, E.; Iritas, I.; Eyup, Y. Keratoconus Could be Associated with Psoriasis: Novel Findings from a Comparative Study. Cornea 2019, 38, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Celik, U.; Aykut, V.; Celik, B.; Tas, M.; Yazgan, S.; Kaldrm, H.; Erdur, S.K. A comparison of corneal biomechanical properties in patients with psoriasis and healthy subjects. Eye Contact Lens. 2015, 41, 127–129. [Google Scholar] [CrossRef]
- Edris, N.; Arfeen, S.; Mosaad, R.; Nassar, G. Evaluation of corneal biomechanical parameters in psoriasis patients: A controlled study. Clin. Ophthalmol. 2020, 14, 1833–1837. [Google Scholar] [CrossRef]


| Characteristics | Non-AA Controls | Alopecia Areata Patients | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||||
| Visual acuity | −0.05 | 0.12 | 0.70 | −0.12 | 0.05 | 0.10 | 0.30 | −0.12 | <0.001 * |
| Cochet–Bonnet aesthesiometry | 5.47 | 0.74 | 4.00 | 6.00 | 3.73 | 1.32 | 0.50 | 6.00 | <0.001 * |
| Axial length | 23.70 | 1.25 | 21.31 | 27.24 | 23.21 | 0.83 | 21.24 | 24.93 | 0.052 |
| Refractive error | −0.56 | 2.64 | −9.75 | 4.75 | −1.16 | 1.66 | −5.75 | 3.00 | 0.027 * |
| Intraocular pressure | 13.74 | 2.90 | 10.00 | 18.00 | 13.91 | 1.72 | 9.00 | 16.00 | 0.618 |
| Conjunctival hyperemia | 0.13 | 0.37 | 0.00 | 2.00 | 0.04 | 0.21 | 0.00 | 1.00 | 0.096 |
| Corneal staining | 0.44 | 0.66 | 0.00 | 2.00 | 0.11 | 0.30 | 0.00 | 1.00 | 0.004 * |
| Cataract | 2.15 | 0.72 | 0.00 | 3.00 | 1.00 | 1.40 | 0.00 | 4.00 | <0.001 * |
| Topographic Variables | Non-AA Controls | Alopecia Areata Patients | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||||
| K1 | 42.79 | 1.57 | 39.70 | 46.50 | 43.44 | 1.07 | 41.50 | 45.50 | 0.019 * |
| K2 | 43.83 | 1.67 | 40.60 | 48.60 | 44.30 | 1.04 | 42.30 | 46.60 | 0.090 |
| Anterior Km | 43.31 | 1.59 | 40.20 | 47.40 | 43.86 | 1.00 | 41.90 | 46.00 | 0.033 * |
| Posterior Km | −6.15 | 0.78 | −6.70 | −0.62 | −6.30 | 0.17 | −6.70 | −6.00 | 0.145 |
| Kmax | 44.55 | 1.83 | 40.90 | 49.60 | 45.11 | 1.10 | 42.70 | 47.10 | 0.040 * |
| ISV | 17.10 | 5.84 | 7.00 | 39.00 | 18.54 | 7.39 | 7.00 | 50.00 | 0.357 |
| IVA | 0.13 | 0.05 | 0.04 | 0.30 | 0.15 | 0.09 | 0.04 | 0.63 | 0.308 |
| KI | 1.01 | 0.02 | 0.97 | 1.05 | 1.02 | 0.03 | 0.94 | 1.15 | 0.007 * |
| CKI | 1.00 | 0.01 | 0.99 | 1.02 | 1.00 | 0.01 | 0.99 | 1.03 | 0.409 |
| IHA | 5.30 | 3.85 | 0.10 | 15.90 | 5.95 | 4.20 | 0.10 | 15.70 | 0.436 |
| IHD | 0.01 | 0.01 | 0.00 | 0.03 | 0.02 | 0.03 | 0.00 | 0.18 | 0.795 |
| Posterior elevation | 8.47 | 4.15 | 2.00 | 25.00 | 10.32 | 5.86 | 2.00 | 35.00 | 0.054 |
| ART-max | 492.21 | 105.63 | 244.00 | 724.00 | 452.44 | 99.49 | 242.00 | 728.00 | 0.026 * |
| BAD-D | 0.77 | 0.61 | −0.44 | 2.12 | 1.25 | 0.68 | −0.41 | 3.01 | <0.001 * |
| Apex corneal thickness | 566.60 | 30.85 | 504.00 | 637.00 | 546.74 | 25.78 | 499.00 | 607.00 | 0.001 * |
| Thinnest corneal thickness | 559.52 | 36.37 | 402.00 | 629.00 | 540.84 | 25.19 | 497.00 | 603.00 | <0.001 * |
| Biomechanical Variables | Non-AA Controls | Alopecia Areata Patients | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||||
| Applanation 1 length | 2.35 | 0.33 | 1.65 | 3.00 | 2.19 | 0.31 | 1.80 | 2.75 | 0.029 * |
| Applanation 1 velocity | 0.14 | 0.02 | 0.10 | 0.18 | 0.15 | 0.02 | 0.09 | 0.18 | 0.415 |
| Applanation 2 length | 2.01 | 0.41 | 0.99 | 3.16 | 1.82 | 0.28 | 1.04 | 2.69 | 0.010 * |
| Applanation 2 velocity | −0.26 | 0.04 | −0.31 | −0.03 | −0.26 | 0.03 | −0.29 | −0.19 | 0.885 |
| Peak distance | 4.91 | 0.28 | 4.20 | 5.49 | 4.87 | 0.26 | 4.16 | 5.26 | 0.458 |
| Concave radius | 6.77 | 0.82 | 3.81 | 8.53 | 6.56 | 0.82 | 3.94 | 9.45 | 0.078 |
| Deformation amplitude | 1.09 | 0.11 | 0.86 | 1.29 | 1.10 | 0.11 | 0.88 | 1.32 | 0.414 |
| CBI | 0.25 | 0.25 | 0.00 | 0.88 | 0.37 | 0.25 | 0.02 | 0.87 | 0.022 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos-Blasco, B.; Burgos-Blasco, P.; Rodriguez-Quet, O.; Arriola-Villalobos, P.; Fernandez-Vigo, J.I.; Saceda-Corralo, D.; Vaño-Galvan, S.; García-Feijóo, J. Alterations in Corneal Sensitivity, Staining and Biomechanics of Alopecia Areata Patients: Novel Findings in a Case-Control Study. J. Clin. Med. 2024, 13, 2426. https://doi.org/10.3390/jcm13082426
Burgos-Blasco B, Burgos-Blasco P, Rodriguez-Quet O, Arriola-Villalobos P, Fernandez-Vigo JI, Saceda-Corralo D, Vaño-Galvan S, García-Feijóo J. Alterations in Corneal Sensitivity, Staining and Biomechanics of Alopecia Areata Patients: Novel Findings in a Case-Control Study. Journal of Clinical Medicine. 2024; 13(8):2426. https://doi.org/10.3390/jcm13082426
Chicago/Turabian StyleBurgos-Blasco, Barbara, Patricia Burgos-Blasco, Olivia Rodriguez-Quet, Pedro Arriola-Villalobos, Jose Ignacio Fernandez-Vigo, David Saceda-Corralo, Sergio Vaño-Galvan, and Julián García-Feijóo. 2024. "Alterations in Corneal Sensitivity, Staining and Biomechanics of Alopecia Areata Patients: Novel Findings in a Case-Control Study" Journal of Clinical Medicine 13, no. 8: 2426. https://doi.org/10.3390/jcm13082426
APA StyleBurgos-Blasco, B., Burgos-Blasco, P., Rodriguez-Quet, O., Arriola-Villalobos, P., Fernandez-Vigo, J. I., Saceda-Corralo, D., Vaño-Galvan, S., & García-Feijóo, J. (2024). Alterations in Corneal Sensitivity, Staining and Biomechanics of Alopecia Areata Patients: Novel Findings in a Case-Control Study. Journal of Clinical Medicine, 13(8), 2426. https://doi.org/10.3390/jcm13082426