Neuroendocrine Disorders in Pediatric Craniopharyngioma Patients
Abstract
:1. Introduction
2. Epidemiology and Pathology
3. Clinical Manifestations at the Time of Diagnosis

4. Imaging Studies
5. Treatment Strategies
5.1. Neurosurgery—Strategies and Effects


5.2. Irradiation
6. Neuroendocrine Sequelae
6.1. Pituitary Deficiencies
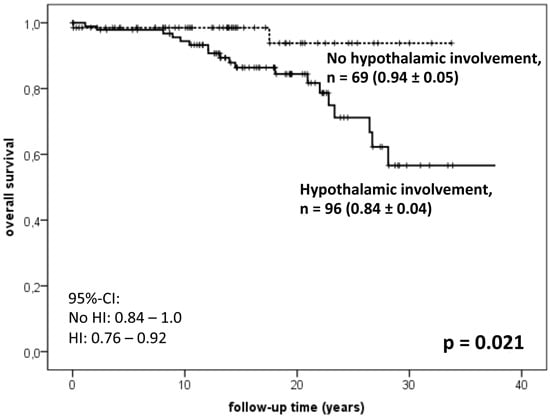
6.2. Hypothalamic Dysfunction
6.3. Obesity and Eating Disorders


6.4. Physical Activity and Energy Expenditure
6.5. Autonomous Nervous System
6.6. Appetite-Regulation
6.7. Treatment of Hypothalamic Obesity
6.9. Survival and Late Mortality
6.10. Treatment Strategies for Prevention of Neuroendocrine Sequelae

| Author (Reference) | n | FU (year) | Grade 0 (0°) | Grade 1 (I°) | Grade 2 (II°) | Treatment Recommendation | Outcome Parameters |
|---|---|---|---|---|---|---|---|
| Puget [38] | 65 | 3 | no HI | HI (distortion/elevation) with negligible hypothalamic damage, the hypothalamus is still visible | tumor spread to the hypothalamus, which was no longer identifiable. | 0°: gross-total resection (GTR) I°: attempt at GTR; if not achieved: 2nd surgery ± XRT II°: subtotal resection with hypothalamic preservation + XRT | lower BMI and similar relapse rate in a prospective cohort treated acc. to algorithm compared with historical cohort |
| Garre [13] | n.a. | n.a. | no HI | according to Puget et al. [38] | according to Puget et al. [38] | 0° + I°: attempt at GTR by experienced surgeon; if not achieved: XRT II°: cyst drainage ± XRT (proton beam therapy at age <5 year) | n.a. |
| Müller [45,46] | 120 | 3 | no HI | HI/lesion of the anterior hypothalamus not involving the MB and the hypothalamic area beyond MB | HI/lesion of the anterior + posterior hypothalamic area, i.e., involving the MB and the area beyond MB | 0°: GTR I°: attempt at GTR; if not achieved: XRT II°: subtotal resection with hypothalamic preservation + XRT | higher BMI and lower QoL in the II° cohort treated by GTR resulting in posterior hypothalamic lesions |
| Flitsch [28] | n.a. | n.a. | no HI | according to Müller et al. [45,46], specifying sections below and above the diaphragm sellae | according to Müller et al. [45,46] | 0°: GTR I°: attempt at GTR—transsphenoidal approach; if not achieved: XRT II°: subtotal resection with hypothalamic preservation—transcranial approach, followed by XRT | n.a. |
| Fjalldall [69] | 42 | 20 | no HI | suprasellar growth, not towards or into the 3rd ventricle (non-TGTV) | suprasellar growth towards or into the 3rd ventricle (TGTV) | Non-TGTV: GTR TGTV: subtotal resection with hypothalamic preservation + XRT | Lower cognitive performance in TGTV patients treated by GTR |
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Wisoff, J.H. Craniopharyngioma. J. Neurosurg. Pediatr. 2008, 1, 124–125. [Google Scholar] [CrossRef]
- Muller, H.L. Childhood craniopharyngioma—Current concepts in diagnosis, therapy and follow-up. Nat. Rev. Endocrinol. 2010, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Childhood craniopharyngioma: Current controversies on management in diagnostics, treatment and follow-up. Expert Rev. Neurother. 2010, 10, 515–524. [Google Scholar] [PubMed]
- Muller, H.L. Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm. Res. 2008, 69, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Karavitaki, N.; Cudlip, S.; Adams, C.B.; Wass, J.A. Craniopharyngiomas. Endocr. Rev. 2006, 27, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Craniopharyngioma. Handb. Clin. Neurol. 2014, 124, 235–253. [Google Scholar]
- Muller, H.L. Craniopharyngioma. Endocr. Rev. 2014, 35, 513–543. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Diagnostics, treatment, and follow-up in craniopharyngioma. Front. Endocrinol. 2011, 2, 70. [Google Scholar] [CrossRef]
- Muller, H.L.; Gebhardt, U.; Etavard-Gorris, N.; Korenke, E.; Warmuth-Metz, M.; Kolb, R.; Sorensen, N.; Calaminus, G. Prognosis and sequela in patients with childhood craniopharyngioma—Results of hit-endo and update on kraniopharyngeom 2000. Klin. Padiatr. 2004, 216, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Childhood craniopharyngioma. Pituitary 2013, 16, 56–67. [Google Scholar] [PubMed]
- Muller, H.L. Paediatrics: Surgical strategy and quality of life in craniopharyngioma. Nat. Rev. Endocrinol. 2013, 9, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Warmth-Metz, M.; Gebhardt, U.; Pietsch, T.; Pohl, F.; Kortmann, R.D.; Calaminus, G.; Muller, H.L. Childhood craniopharyngioma—Changes of treatment strategies in the trials kraniopharyngeom 2000/2007. Klin. Padiatr. 2014, 226, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Garre, M.L.; Cama, A. Craniopharyngioma: Modern concepts in pathogenesis and treatment. Curr. Opin. Pediatr. 2007, 19, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.H.; Feldt-Rasmussen, U.; Poulsgaard, L.; Kristensen, L.O.; Astrup, J.; Jorgensen, J.O.; Bjerre, P.; Andersen, M.; Andersen, C.; Jorgensen, J.; et al. Incidence of craniopharyngioma in denmark (n = 189) and estimated world incidence of craniopharyngioma in children and adults. J. NeuroOncol. 2011, 104, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Bunin, G.R.; Surawicz, T.S.; Witman, P.A.; Preston-Martin, S.; Davis, F.; Bruner, J.M. The descriptive epidemiology of craniopharyngioma. J. Neurosurg. 1998, 89, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Muller-Scholden, J.; Lehrnbecher, T.; Muller, H.L.; Bensch, J.; Hengen, R.H.; Sorensen, N.; Stockhausen, H.B. Radical surgery in a neonate with craniopharyngioma. Report of a case. Pediatr. Neurosurg. 2000, 33, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S.J.; Ansorge, O. Pathology and pathogenesis of craniopharyngiomas. Pituitary 2013, 16, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Holsken, A.; Buchfelder, M.; Fahlbusch, R.; Blumcke, I.; Buslei, R. Tumour cell migration in adamantinomatous craniopharyngiomas is promoted by activated wnt-signalling. Acta Neuropathol. 2010, 119, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Gaston-Massuet, C.; Andoniadou, C.L.; Signore, M.; Jayakody, S.A.; Charolidi, N.; Kyeyune, R.; Vernay, B.; Jacques, T.S.; Taketo, M.M.; Le Tissier, P.; et al. Increased wingless (wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 11482–11487. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.L.; Kaatsch, P.; Warmuth-Metz, M.; Flentje, M.; Sörensen, N. Kraniopharyngeom im KIndes- und Jugendalter—Diagnostische und therapeutische Strategien. Monatsschrift Kinderheilkd. 2003, 151, 1056–1063. [Google Scholar] [CrossRef]
- Muller, H.L.; Emser, A.; Faldum, A.; Bruhnken, G.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sorensen, N. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 2004, 89, 3298–3305. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, M.; Massimi, L.; Tamburrini, G.; Cappa, M.; Di Rocco, C. Long-term results of the surgical treatment of craniopharyngioma: The experience at the policlinico gemelli, catholic university, Rome. Childs Nerv. Syst. ChNS 2005, 21, 747–757. [Google Scholar] [CrossRef]
- Hoffman, H.J.; De Silva, M.; Humphreys, R.P.; Drake, J.M.; Smith, M.L.; Blaser, S.I. Aggressive surgical management of craniopharyngiomas in children. J. Neurosurg. 1992, 76, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.E.; Wisoff, J.H. Surgical management of giant pediatric craniopharyngiomas. J. Neurosurg. Pediatr. 2010, 6, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Warmuth-Metz, M.; Gnekow, A.K.; Muller, H.; Solymosi, L. Differential diagnosis of suprasellar tumors in children. Klinische Padiatr. 2004, 216, 323–330. [Google Scholar] [CrossRef]
- Muller, H.L. Craniopharyngioma—A childhood and adult disease with challenging characteristics. Front. Endocrinol. 2012, 3, 80. [Google Scholar] [CrossRef]
- Choux, M.; Lena, G. Bases of surgical management of craniopharyngioma in children. Acta Neurochir. Suppl. 1979, 28, 348. [Google Scholar] [PubMed]
- Flitsch, J.; Muller, H.L.; Burkhardt, T. Surgical strategies in childhood craniopharyngioma. Front. Endocrinol. 2011, 2, 96. [Google Scholar] [CrossRef]
- Fahlbusch, R.; Honegger, J.; Paulus, W.; Huk, W.; Buchfelder, M. Surgical treatment of craniopharyngiomas: Experience with 168 patients. J. Neurosurg. 1999, 90, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Consequences of craniopharyngioma surgery in children. J. Clin. Endocrinol. Metab. 2011, 96, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Buchfelder, M.; Schlaffer, S.M.; Lin, F.; Kleindienst, A. Surgery for craniopharyngioma. Pituitary 2013, 16, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. More or less? Treatment strategies in childhood craniopharyngioma. Childs Nerv. Syst. ChNS 2006, 22, 156–157. [Google Scholar] [CrossRef]
- Muller, H.L.; Gebhardt, U.; Pohl, F.; Flentje, M.; Emser, A.; Warmuth-Metz, M.; Kolb, R.; Calaminus, G.; Sorensen, N. Relapse pattern after complete resection and early progression after incomplete resection of childhood craniopharyngioma. Klinische Padiatr. 2006, 218, 315–320. [Google Scholar] [CrossRef]
- Kordes, U.; Flitsch, J.; Hagel, C.; Goebell, E.; Schwarz, R.; Herberhold, T.; von Bueren, A.O.; Rutkowski, S.; Muller, H.L. Ectopic craniopharyngioma. Klinische Padiatr. 2011, 223, 176–177. [Google Scholar] [CrossRef]
- Becker, G.; Kortmann, R.D.; Skalej, M.; Bamberg, M. The role of radiotherapy in the treatment of craniopharyngioma—Indications, results, side effects. Front. Radiat. Ther. Oncol. 1999, 33, 100–113. [Google Scholar] [PubMed]
- Elowe-Gruau, E.; Beltrand, J.; Brauner, R.; Pinto, G.; Samara-Boustani, D.; Thalassinos, C.; Busiah, K.; Laborde, K.; Boddaert, N.; Zerah, M.; et al. Childhood craniopharyngioma: Hypothalamus-sparing surgery decreases the risk of obesity. J. Clin. Endocrinol. Metab. 2013, 98, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.E.; Jane, J.A., Jr.; Wisoff, J.H. Surgical management of craniopharyngiomas in children: Meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 2011, 69, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Puget, S.; Grill, J.; Habrand, J.L.; Sainte-Rose, C. Multimodal treatment of craniopharyngioma: Defining a risk-adapted strategy. J. Pediatr. Endocrinol. Metab. JPEM 2006, 19 (Suppl. S1), 367–370. [Google Scholar]
- Karavitaki, N.; Brufani, C.; Warner, J.T.; Adams, C.B.; Richards, P.; Ansorge, O.; Shine, B.; Turner, H.E.; Wass, J.A. Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. 2005, 62, 397–409. [Google Scholar] [CrossRef]
- Vinchon, M.; Weill, J.; Delestret, I.; Dhellemmes, P. Craniopharyngioma and hypothalamic obesity in children. Childs Nerv. Syst. ChNS 2009, 25, 347–352. [Google Scholar] [CrossRef]
- Hetelekidis, S.; Barnes, P.D.; Tao, M.L.; Fischer, E.G.; Schneider, L.; Scott, R.M.; Tarbell, N.J. 20-Year experience in childhood craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Rajan, B.; Ashley, S.; Gorman, C.; Jose, C.C.; Horwich, A.; Bloom, H.J.; Marsh, H.; Brada, M. Craniopharyngioma—A long-term results following limited surgery and radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1993, 26, 1–10. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Sanford, R.A.; Mulhern, R.K.; Thompson, S.J.; Wilson, M.W.; Lustig, R.H.; Kun, L.E. Craniopharyngioma: The St. Jude children’s research hospital experience 1984–2001. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Sanford, R.A. Craniopharyngioma: Results of survey of the american society of pediatric neurosurgery. Pediatr. Neurosurg. 1994, 21 (Suppl. S1), 39–43. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Gebhardt, U.; Faldum, A.; Warmuth-Metz, M.; Pietsch, T.; Pohl, F.; Calaminus, G.; Sorensen, N. Xanthogranuloma, rathke’s cyst, and childhood craniopharyngioma: Results of prospective multinational studies of children and adolescents with rare sellar malformations. J. Clin. Endocrinol. Metab. 2012, 97, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Gebhardt, U.; Teske, C.; Faldum, A.; Zwiener, I.; Warmuth-Metz, M.; Pietsch, T.; Pohl, F.; Sorensen, N.; Calaminus, G. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: Results of the multinational prospective trial kraniopharyngeom 2000 after 3-year follow-up. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2011, 165, 17–24. [Google Scholar] [CrossRef]
- Daubenbüchel, A.M.M.; Hoffmann, A.; Gebhardt, U.; Warmuth-Metz, M.; Sterkenburg, A.S.; Müller, H.L. Hydrocephalus and hypothalamic involvement in pediatric patients with craniopharyngioma or cysts of Rathkes pouch—Impact on long-term prognosis. Eur. J. Endocrinol. 2015, in press. [Google Scholar]
- Habrand, J.L.; Saran, F.; Alapetite, C.; Noel, G.; El Boustany, R.; Grill, J. Radiation therapy in the management of craniopharyngioma: Current concepts and future developments. J. Pediatr. Endocrinol. Metab. JPEM 2006, 19 (Suppl. S1), 389–394. [Google Scholar]
- Minniti, G.; Esposito, V.; Amichetti, M.; Enrici, R.M. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg. Rev. 2009, 32, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kortmann, R.D. Different approaches in radiation therapy of craniopharyngioma. Front. Endocrinol. 2011, 2, 100. [Google Scholar] [CrossRef]
- Aggarwal, A.; Fersht, N.; Brada, M. Radiotherapy for craniopharyngioma. Pituitary 2013, 16, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Kiehna, E.N.; Kun, L.E.; Mulhern, R.K.; Li, C.; Xiong, X.; Boop, F.A.; Sanford, R.A. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J. Neurosurg. 2006, 104, 94–102. [Google Scholar] [PubMed]
- Boehling, N.S.; Grosshans, D.R.; Bluett, J.B.; Palmer, M.T.; Song, X.; Amos, R.A.; Sahoo, N.; Meyer, J.J.; Mahajan, A.; Woo, S.Y. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Beltran, C.; Roca, M.; Merchant, T.E. On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 281–287. [Google Scholar] [CrossRef]
- Honegger, J.; Buchfelder, M.; Fahlbusch, R. Surgical treatment of craniopharyngiomas: Endocrinological results. J. Neurosurg. 1999, 90, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Steno, J.; Bizik, I.; Steno, A.; Matejcik, V. Craniopharyngiomas in children: How radical should the surgeon be? Childs Nerv. Syst. ChNS 2011, 27, 41–54. [Google Scholar] [CrossRef]
- Jung, T.Y.; Jung, S.; Moon, K.S.; Kim, I.Y.; Kang, S.S.; Kim, J.H. Endocrinological outcomes of pediatric craniopharyngiomas with anatomical pituitary stalk preservation: Preliminary study. Pediatr. Neurosurg. 2010, 46, 205–212. [Google Scholar] [CrossRef] [PubMed]
- De Vile, C.J.; Grant, D.B.; Kendall, B.E.; Neville, B.G.; Stanhope, R.; Watkins, K.E.; Hayward, R.D. Management of childhood craniopharyngioma: Can the morbidity of radical surgery be predicted? J. Neurosurg. 1996, 85, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Poretti, A.; Grotzer, M.A.; Ribi, K.; Schonle, E.; Boltshauser, E. Outcome of craniopharyngioma in children: Long-term complications and quality of life. Dev. Med. Child neurol. 2004, 46, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ahmet, A.; Blaser, S.; Stephens, D.; Guger, S.; Rutkas, J.T.; Hamilton, J. Weight gain in craniopharyngioma—A model for hypothalamic obesity. J. Pediatr. Endocrinol. Metab. JPEM 2006, 19, 121–127. [Google Scholar]
- Halac, I.; Zimmerman, D. Endocrine manifestations of craniopharyngioma. Childs Nerv. Syst. ChNS 2005, 21, 640–648. [Google Scholar] [CrossRef]
- Crom, D.; Smith, D.; Xiong, Z.; Onar, A.; Hudson, M.; Merchant, T.; Morris, E. Health status in long-term survivors of pediatric craniopharyngiomas. Am. Assoc. Neurosci. Nurses 2010, 42, 323–328. [Google Scholar] [CrossRef]
- Geffner, M.; Lundberg, M.; Koltowska-Haggstrom, M.; Abs, R.; Verhelst, J.; Erfurth, E.M.; Kendall-Taylor, P.; Price, D.A.; Jonsson, P.; Bakker, B. Changes in height, weight, and body mass index in children with craniopharyngioma after three years of growth hormone therapy: Analysis of kigs (pfizer international growth database). J. Clin. Endocrinol. Metab. 2004, 89, 5435–5440. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Ogle, G.D.; Garnett, S.P.; Briody, J.N.; Lee, J.W.; Cowell, C.T. Features of the metabolic syndrome after childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 2004, 89, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Matson, D.D. Craniopharyngioma. Clin. Neurosurg. 1964, 10, 14. [Google Scholar]
- De Vile, C.J.; Grant, D.B.; Hayward, R.D.; Kendall, B.E.; Neville, B.G.; Stanhope, R. Obesity in childhood craniopharyngioma: Relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J. Clin. Endocrinol. Metab. 1996, 81, 2734–2737. [Google Scholar]
- Holmer, H.; Pozarek, G.; Wirfalt, E.; Popovic, V.; Ekman, B.; Bjork, J.; Erfurth, E.M. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J. Clin. Endocrinol. Metab. 2010, 95, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Holmer, H.; Ekman, B.; Bjork, J.; Nordstom, C.H.; Popovic, V.; Siversson, A.; Erfurth, E.M. Hypothalamic involvement predicts cardiovascular risk in adults with childhood onset craniopharyngioma on long-term GH therapy. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2009, 161, 671–679. [Google Scholar] [CrossRef]
- Fjalldal, S.; Holmer, H.; Rylander, L.; Elfving, M.; Ekman, B.; Osterberg, K.; Erfurth, E.M. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 2013, 98, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Handwerker, G.; Wollny, B.; Faldum, A.; Sorensen, N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J. Clin. Endocrinol. Metab. 2002, 87, 3993–3996. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Bueb, K.; Bartels, U.; Roth, C.; Harz, K.; Graf, N.; Korinthenberg, R.; Bettendorf, M.; Kuhl, J.; Gutjahr, P.; et al. Obesity after childhood craniopharyngioma—German multicenter study on pre-operative risk factors and quality of life. Klinische Padiatr. 2001, 213, 244–249. [Google Scholar] [CrossRef]
- O’Gorman, C.S.; Simoneau-Roy, J.; Pencharz, P.; MacFarlane, J.; MacLusky, I.; Narang, I.; Adeli, K.; Daneman, D.; Hamilton, J. Sleep-disordered breathing is increased in obese adolescents with craniopharyngioma compared with obese controls. J. Clin. Endocrinol. Metab. 2010, 95, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Heinrich, M.; Bueb, K.; Etavard-Gorris, N.; Gebhardt, U.; Kolb, R.; Sorensen, N. Perioperative dexamethasone treatment in childhood craniopharyngioma—Influence on short-term and long-term weight gain. Exp. Clin. Endocrinol. Diabetes 2003, 111, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Lek, N.; Prentice, P.; Williams, R.M.; Ong, K.K.; Burke, G.A.; Acerini, C.L. Risk factors for obesity in childhood survivors of suprasellar brain tumours: A retrospective study. Acta Paediatr. 2010, 99, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Mong, S.; Pomeroy, S.L.; Cecchin, F.; Juraszek, A.; Alexander, M.E. Cardiac risk after craniopharyngioma therapy. Pediatr. Neurol. 2008, 38, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Schmid, E.M.; Schutte, P.J.; Voormolen, J.H.; Biermasz, N.R.; van Thiel, S.W.; Corssmit, E.P.; Smit, J.W.; Roelfsema, F.; Romijn, J.A. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin. Endocrinol. 2005, 62, 197–204. [Google Scholar] [CrossRef]
- Visser, J.; Hukin, J.; Sargent, M.; Steinbok, P.; Goddard, K.; Fryer, C. Late mortality in pediatric patients with craniopharyngioma. J. Neurooncol. 2010, 100, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Bowman, R.M. Craniopharyngiomas in children: Surgical experience at children’s memorial hospital. Childs Nerv. Syst. ChNS 2005, 21, 729–746. [Google Scholar] [CrossRef]
- Fisher, P.G.; Jenab, J.; Gopldthwaite, P.T.; Tihan, T.; Wharam, M.D.; Foer, D.R.; Burger, P.C. Outcomes and failure patterns in childhood craniopharyngiomas. Childs Nerv. Syst. ChNS 1998, 14, 558–563. [Google Scholar] [CrossRef]
- Lin, L.L.; El Naqa, I.; Leonard, J.R.; Park, T.S.; Hollander, A.S.; Michalski, J.M.; Mansur, D.B. Long-term outcome in children treated for craniopharyngioma with and without radiotherapy. J. Neurosurg. Pediatr. 2008, 1, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kalapurakal, J.A.; Goldman, S.; Hsieh, Y.C.; Tomita, T.; Marymont, M.H. Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med. Pediatr. Oncol. 2003, 40, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.M.; Hetelekidis, S.; Barnes, P.D.; Goumnerova, L.; Tarbell, N.J. Surgery, radiation, and combination therapy in the treatment of childhood craniopharyngioma—A 20-year experience. Pediatr. Neurosurg. 1994, 21 (Suppl. 1), 75–81. [Google Scholar] [CrossRef] [PubMed]
- Khafaga, Y.; Jenkin, D.; Kanaan, I.; Hassounah, M.; Al Shabanah, M.; Gray, A. Craniopharyngioma in children. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Post, S.R.; Srivannaboon, K.; Rose, S.R.; Danish, R.K.; Burghen, G.A.; Xiong, X.; Wu, S.; Merchant, T.E. Risk factors for the development of obesity in children surviving brain tumors. J. Clin. Endocrinol. Metab. 2003, 88, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Gooren, L.J.; Hofman, M.A. The human hypothalamus in relation to gender and sexual orientation. Prog. Brain Res. 1992, 93, 205–217. [Google Scholar] [PubMed]
- Kreier, F.; Fliers, E.; Voshol, P.J.; Van Eden, C.G.; Havekes, L.M.; Kalsbeek, A.; Van Heijningen, C.L.; Sluiter, A.A.; Mettenleiter, T.C.; Romijn, J.A.; et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat—Functional implications. J. Clin. Investig. 2002, 110, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Wilken, B.; Hanefeld, F.; Schroter, W.; Leonhardt, U. Hyperphagia in children with craniopharyngioma is associated with hyperleptinaemia and a failure in the downregulation of appetite. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 1998, 138, 89–91. [Google Scholar] [CrossRef]
- Harz, K.J.; Muller, H.L.; Waldeck, E.; Pudel, V.; Roth, C. Obesity in patients with craniopharyngioma: Assessment of food intake and movement counts indicating physical activity. J. Clin. Endocrinol. Metab. 2003, 88, 5227–5231. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Handwerker, G.; Gebhardt, U.; Faldum, A.; Emser, A.; Kolb, R.; Sorensen, N. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control CCC 2006, 17, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Muller-Stover, S.; Gebhardt, U.; Kolb, R.; Sorensen, N.; Handwerker, G. Secondary narcolepsy may be a causative factor of increased daytime sleepiness in obese childhood craniopharyngioma patients. J. Pediatr. Endocrinol. Metab. JPEM 2006, 19 (Suppl. S1), 423–429. [Google Scholar]
- Muller, H.L. Increased daytime sleepiness in patients with childhood craniopharyngioma and hypothalamic tumor involvement: Review of the literature and perspectives. Int. J. Endocrinol. 2010, 2010, 519607. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.W.; Krawiecki, N.; Meacham, L.R. The use of dextroamphetamine to treat obesity and hyperphagia in children treated for craniopharyngioma. Arch. Pediatr. Adolesc. Med. 2002, 156, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.G.; Grundy, R.G.; Kirk, J.M. Reductions in basal metabolic rate and physical activity contribute to hypothalamic obesity. J. Clin. Endocrinol. Metab. 2008, 93, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Shah, R.; Tershakovec, A.M.; Zemel, B.S.; Sutton, L.N.; Grimberg, A.; Moshang, T. Energy expenditure in obesity associated with craniopharyngioma. Childs Nerv. Syst. ChNS 2010, 26, 913–917. [Google Scholar] [CrossRef]
- Lustig, R.H.; Hinds, P.S.; Ringwald-Smith, K.; Christensen, R.K.; Kaste, S.C.; Schreiber, R.E.; Rai, S.N.; Lensing, S.Y.; Wu, S.; Xiong, X. Octreotide therapy of pediatric hypothalamic obesity: A double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2003, 88, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Hypothalamic obesity after craniopharyngioma: Mechanisms, diagnosis, and treatment. Front. Endocrinol. 2011, 2, 60. [Google Scholar] [CrossRef]
- Roth, C.L.; Hunneman, D.H.; Gebhardt, U.; Stoffel-Wagner, B.; Reinehr, T.; Muller, H.L. Reduced sympathetic metabolites in urine of obese patients with craniopharyngioma. Pediatr. Res. 2007, 61, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Syme, C.; McCrindle, B.W.; Hamilton, J. Autonomic nervous system balance in children and adolescents with craniopharyngioma and hypothalamic obesity. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2013, 168, 845–852. [Google Scholar] [CrossRef]
- Roth, C.L. Hypothalamic obesity in patients with craniopharyngioma: Profound changes of several weight regulatory circuits. Front. Endocrinol. 2011, 2, 49. [Google Scholar] [CrossRef]
- Roth, C.L.; Gebhardt, U.; Muller, H.L. Appetite-regulating hormone changes in patients with craniopharyngioma. Obesity (Silver Spring) 2011, 19, 36–42. [Google Scholar] [CrossRef]
- Roth, C.L.; Enriori, P.J.; Gebhardt, U.; Hinney, A.; Muller, H.L.; Hebebrand, J.; Reinehr, T.; Cowley, M.A. Changes of peripheral alpha-melanocyte-stimulating hormone in childhood obesity. Metab. Clin. Exp. 2010, 59, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Aylward, E.; Liang, O.; Kleinhans, N.M.; Pauley, G.; Schur, E.A. Functional neuroimaging in craniopharyngioma: A useful tool to better understand hypothalamic obesity? Obes. Facts 2012, 5, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Sterkenburg, A.S.; Hoffmann, A.; Gebhardt, U.; Waldeck, E.; Springer, S.; Muller, H.L. Childhood craniopharyngioma with hypothalamic obesity—No long-term weight reduction due to rehabilitation programs. Klinische Padiatr. 2014, 226, 344–350. [Google Scholar] [CrossRef]
- Schofl, C.; Schleth, A.; Berger, D.; Terkamp, C.; von zur Muhlen, A.; Brabant, G. Sympathoadrenal counterregulation in patients with hypothalamic craniopharyngioma. J. Clin. Endocrinol. Metab. 2002, 87, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Coutant, R.; Maurey, H.; Rouleau, S.; Mathieu, E.; Mercier, P.; Limal, J.M.; Le Bouil, A. Defect in epinephrine production in children with craniopharyngioma: Functional or organic origin? J. Clin. Endocrinol. Metab. 2003, 88, 5969–5975. [Google Scholar] [CrossRef] [PubMed]
- Ismail, D.; O’Connell, M.A.; Zacharin, M.R. Dexamphetamine use for management of obesity and hypersomnolence following hypothalamic injury. J. Pediatr. Endocrinol. Metab. JPEM 2006, 19, 129–134. [Google Scholar] [CrossRef]
- Elfers, C.T.; Roth, C.L. Effects of methylphenidate on weight gain and food intake in hypothalamic obesity. Front. Endocrinol. 2011, 2, 78. [Google Scholar] [CrossRef]
- Lustig, R.H. Hypothalamic obesity: Causes, consequences, treatment. Pediatr. Endocrinol. Rev. PER 2008, 6, 220–227. [Google Scholar]
- Clinical Trials.gov. Available online: http://clinicaltrials.gov/ct2/show/NCT00076362 (accessed on 1 August 2014).
- Muller, H.L.; Gebhardt, U.; Wessel, V.; Schroder, S.; Kolb, R.; Sorensen, N.; Maroske, J.; Hanisch, E. First experiences with laparoscopic adjustable gastric banding (lagb) in the treatment of patients with childhood craniopharyngioma and morbid obesity. Klinische Padiatr. 2007, 219, 323–325. [Google Scholar] [CrossRef]
- Inge, T.H.; Pfluger, P.; Zeller, M.; Rose, S.R.; Burget, L.; Sundararajan, S.; Daniels, S.R.; Tschop, M.H. Gastric bypass surgery for treatment of hypothalamic obesity after craniopharyngioma therapy. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Bingham, N.C.; Rose, S.R.; Inge, T.H. Bariatric surgery in hypothalamic obesity. Front. Endocrinol. 2012, 3, 23. [Google Scholar] [CrossRef]
- Muller, H.L.; Gebhardt, U.; Maroske, J.; Hanisch, E. Long-term follow-up of morbidly obese patients with childhood craniopharyngioma after laparoscopic adjustable gastric banding (lagb). Klinische Padiatr. 2011, 223, 372–373. [Google Scholar] [CrossRef]
- Bretault, M.; Boillot, A.; Muzard, L.; Poitou, C.; Oppert, J.M.; Barsamian, C.; Gatta, B.; Muller, H.; Weismann, D.; Rottembourg, D.; et al. Bariatric surgery following treatment for craniopharyngioma: A systematic review and individual-level data meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Schultes, B.; Ernst, B.; Schmid, F.; Thurnheer, M. Distal gastric bypass surgery for the treatment of hypothalamic obesity after childhood craniopharyngioma. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2009, 161, 201–206. [Google Scholar] [CrossRef]
- Rottembourg, D.; O’Gorman, C.S.; Urbach, S.; Garneau, P.Y.; Langer, J.C.; Van Vliet, G.; Hamilton, J.; Huot, C. Outcome after bariatric surgery in two adolescents with hypothalamic obesity following treatment of craniopharyngioma. J. Pediatr. Endocrinol. Metab. JPEM 2009, 22, 867–872. [Google Scholar]
- Bereket, A.; Kiess, W.; Lustig, R.H.; Muller, H.L.; Goldstone, A.P.; Weiss, R.; Yavuz, Y.; Hochberg, Z. Hypothalamic obesity in children. Obes. Rev. 2012, 13, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Van Effenterre, R.; Boch, A.L. Craniopharyngioma in adults and children: A study of 122 surgical cases. J. Neurosurg. 2002, 97, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Faldum, A.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sorensen, N. Functional capacity, obesity and hypothalamic involvement: Cross-sectional study on 212 patients with childhood craniopharyngioma. Klinische Padiatr. 2003, 215, 310–314. [Google Scholar] [CrossRef]
- Muller, H.L.; Bruhnken, G.; Emser, A.; Faldum, A.; Etavard-Gorris, N.; Gebhardt, U.; Kolb, R.; Sorensen, N. Longitudinal study on quality of life in 102 survivors of childhood craniopharyngioma. Childs Nerv. Syst. ChNS 2005, 21, 975–980. [Google Scholar] [CrossRef]
- Ondruch, A.; Maryniak, A.; Kropiwnicki, T.; Roszkowski, M.; Daszkiewicz, P. Cognitive and social functioning in children and adolescents after the removal of craniopharyngioma. Childs Nerv. Syst. 2011, 27, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Postma, F.P.; Sterkenburg, A.S.; Gebhardt, U.; Muller, H.L. Eating behavior, weight problems and eating disorders in 101 long-term survivors of childhood-onset craniopharyngioma. J. Pediatr. Endocrinol. Metab. JPEM 2015, 28, 35–43. [Google Scholar]
- Cavazzuti, V.; Fischer, E.; Welch, K.; Belli, J.; Winston, K. Neurological and psychophysiological sequelae following different treatments of craniopharyngioma in children. J. Neurosurg. 1983, 59, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Riva, D.; Pantaleoni, C.; Devoti, M.; Saletti, V.; Nichelli, F.; Giorgi, C. Late neuropsychological and behavioral outcome of children surgically treated for craniopharyngioma. Childs Nerv. Syst. 1998, 14, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kiehna, E.; Mulhern, R.; Li, C.; Xiong, X.; Merchant, T. Changes in attentional performance of children and young adults iwth localized primary brain tumors after conformal radiation therapy. J. Clin. Oncol. 2006, 24, 5283–5290. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Waber, D.; Scott, R.; Goumnerova, L.; Kieran, M.; Cohen, L.; Kim, F.; Billett, A.; Tarbell, N.; Pomeroy, S. Memory deficits among children with craniopharyngioma. Neurosurgery 2001, 49, 1053–1058. [Google Scholar] [PubMed]
- Sands, S.; Milner, J.; Goldberg, J.; Mukhi, V.; Moliterno, J.; Maxfield, C.; Wisoff, J. Quality of life and behavioral follow-up study of pediatric survivors of cranipharyngioma. J. Neurosurg. (Pediatrics) 2005, 103, 302–311. [Google Scholar] [CrossRef]
- Ozyurt, J.; Thiel, C.M.; Lorenzen, A.; Gebhardt, U.; Calaminus, G.; Warmuth-Metz, M.; Muller, H.L. Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. J. Pediatr. 2014, 164, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, J.; Lorenzen, A.; Gebhardt, U.; Warmuth-Metz, M.; Muller, H.L.; Thiel, C.M. Remote effects of hypothalamic lesions in the prefrontal cortex of craniopharygioma patients. Neurobiol. Learn. Mem. 2014, 111, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.; Ambrosio, A.; Ambrosio, C. Neurological and behavioral sequelae following different approaches to craniopharyngioma. Long-term follow-up review and therapeutic guidelines. Childs Nerv. Syst. 1990, 6, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Erfurth, E.M.; Holmer, H.; Fjalldal, S.B. Mortality and morbidity in adult craniopharyngioma. Pituitary 2013, 16, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Stripp, D.C.; Maity, A.; Janss, A.J.; Belasco, J.B.; Tochner, Z.A.; Goldwein, J.W.; Moshang, T.; Rorke, L.B.; Phillips, P.C.; Sutton, L.N.; et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Bartels, U.; Laperriere, N.; Bouffet, E.; Drake, J. Intracystic therapies for cystic craniopharyngioma in childhood. Front. Endocrinol. 2012, 3, 39. [Google Scholar] [CrossRef]
- Cohen, M.; Bartels, U.; Branson, H.; Kulkarni, A.V.; Hamilton, J. Trends in treatment and outcomes of pediatric craniopharyngioma, 1975–2011. Neurooncology 2013, 15, 767–774. [Google Scholar]
- McLaughlin, N.; Laws, E.R.; Oyesiku, N.M.; Katznelson, L.; Kelly, D.F. Pituitary centers of excellence. Neurosurgery 2012, 71, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Childhood craniopharyngioma—Current status and recent perspectives in diagnostics and treatment. J. Pediatr. Endocrinol. Metab. JPEM 2015, 28, 1–2. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daubenbüchel, A.M.M.; Müller, H.L. Neuroendocrine Disorders in Pediatric Craniopharyngioma Patients. J. Clin. Med. 2015, 4, 389-413. https://doi.org/10.3390/jcm4030389
Daubenbüchel AMM, Müller HL. Neuroendocrine Disorders in Pediatric Craniopharyngioma Patients. Journal of Clinical Medicine. 2015; 4(3):389-413. https://doi.org/10.3390/jcm4030389
Chicago/Turabian StyleDaubenbüchel, Anna M. M., and Hermann L. Müller. 2015. "Neuroendocrine Disorders in Pediatric Craniopharyngioma Patients" Journal of Clinical Medicine 4, no. 3: 389-413. https://doi.org/10.3390/jcm4030389
APA StyleDaubenbüchel, A. M. M., & Müller, H. L. (2015). Neuroendocrine Disorders in Pediatric Craniopharyngioma Patients. Journal of Clinical Medicine, 4(3), 389-413. https://doi.org/10.3390/jcm4030389