Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat
Abstract
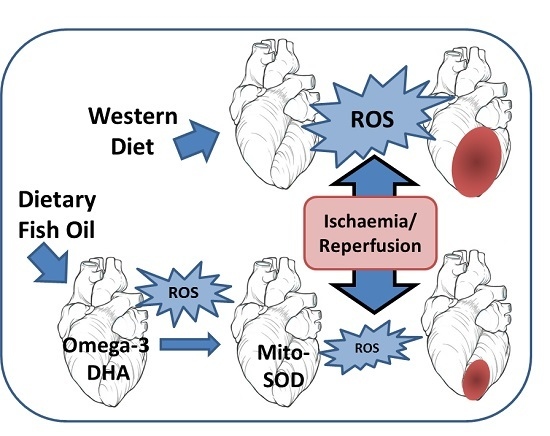
:1. Introduction
2. Experimental Section
2.1. Animals and Diets
2.2. Heart Preparation
2.3. Index Ischaemia and Ischaemic Preconditioning
- Control normoxia protocol (n = 6 per diet): Hearts were perfused throughout with oxygenated Krebs–Henseleit solution.
- Ischaemia protocol (n = 6 per diet): Hearts were normoxically perfused for 30 min followed by 30 min index-ischaemia and 120 min normoxic reperfusion. Index-ischaemia was induced by occluding the left anterior descending coronary artery.
- Ischaemic preconditioning (IPC) protocol (n = 6 per diet): Hearts were subjected to three cycles of five minutes global ischaemia (zero perfusion), each followed by five minutes normoxic reperfusion, prior to the 30 min index-ischaemia then 120 min normoxic reperfusion [5].
2.4. Measurement of Oxidative Stress Biomarkers
2.5. Measurement of Antioxidants
2.6. Myocardial Fatty Acid Analyses
2.7. Statistical Analyses
3. Results
3.1. Myocardial Membrane Phospholipid Fatty Acid Composition
3.2. Basal Properties: Effects of Diet on Oxidative Stress and Antioxidant Activity
3.3. Ischaemic Responses: Effects of Diet and Ischaemic Preconditioning on Oxidative Stress and Antioxidant Capacity in Hearts Subjected to Regional I/R
3.4. Infarct
3.5. Associations between Oxidation Biomarkers, Antioxidant and Infarct Size
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trikalinos, T.A.; Lee, J.; Moorthy, D.; Yu, W.W.; Lau, J.; Lichtenstein, A.H.; Chung, M. Effects of eicosapentanoic acid and docosahexanoic acid on mortality across diverse settings: Systematic review and meta-analysis of randomized trials and prospective cohorts. Available online: http://www.ncbi.nlm.nih.gov/books/NBK91413/pdf/Bookshelf_NBK91413.pdf (accessed on 2 March 2016).
- Valagussa, F.; Franzosi, M.G.; Geraci, E.; Mininni, N.; Nicolosi, G.L.; Santini, M.; Tavazzi, L.; Vecchio, C.; Marchioli, R.; Bomba, E.; et al. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health—Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.L. Cardiac physiology and clinical efficacy of dietary fish oil clarified through cellular mechanisms of omega-3 polyunsaturated fatty acids. Eur. J. Appl. Physiol. 2014, 114, 1333–1356. [Google Scholar] [CrossRef] [PubMed]
- Abdukeyum, G.G.; Owen, A.J.; McLennan, P.L. Dietary (n-3) long-chain polyunsaturated fatty acids inhibit ischemia and reperfusion arrhythmias and infarction in rat heart not enhanced by ischemic preconditioning. J. Nutr. 2008, 138, 1902–1909. [Google Scholar] [PubMed]
- Zeghichi-Hamri, S.; de Lorgeril, M.; Salen, P.; Chibane, M.; de Leiris, J.; Boucher, F.; Laporte, F. Protective effect of dietary n-3 polyunsaturated fatty acids on myocardial resistance to ischemia-reperfusion injury in rats. Nutr. Res. 2010, 30, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.V.; Yang, X.M.; Downey, J.M. Conscious rabbits become tolerant to multiple episodes of ischemic preconditioning. Circ. Res. 1994, 74, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.L.; Abeywardena, M.Y.; Charnock, J.S. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am. Heart J. 1988, 116, 709–717. [Google Scholar] [CrossRef]
- McLennan, P.L. Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models. Lipids 2001, 36, S111–S114. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.; Howe, P.; Abeywardena, M.; Muggli, R.; Raederstorff, D.; Mano, M.; Rayner, T.; Head, R. The cardiovascular protective role of docosahexaenoic acid. Eur. J. Pharmacol. 1996, 300, 83–89. [Google Scholar] [CrossRef]
- Dana, A.; Baxter, G.F.; Walker, J.M.; Yellon, D.M. Prolonging the delayed phase of myocardial protection: Repetitive adenosine A(1) receptor activation maintains rabbit myocardium in a preconditioned state. J. Am. Coll. Cardiol. 1998, 31, 1142–1149. [Google Scholar] [CrossRef]
- Hoshida, S.; Yamashita, N.; Otsu, K.; Hori, M. Repeated physiologic stresses provide persistent cardioprotection against ischemia-reperfusion injury in rats. J. Am. Coll. Cardiol. 2002, 40, 826–831. [Google Scholar] [CrossRef]
- Marber, M.S.; Latchman, D.S.; Walker, J.M.; Yellon, D.M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 1993, 88, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R. The early and late phases of preconditioning against myocardial stunning and the essential role of oxyradicals in the late phase: An overview. Basic Res. Cardiol. 1996, 91, 57–63. [Google Scholar] [PubMed]
- Baxter, G.F.; Ferdinandy, P. Delayed preconditioning of myocardium: Current perspectives. Basic Res. Cardiol. 2001, 96, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.B.; Zhai, X.L.; Ashraf, M. Direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocytes. Circulation 1996, 93, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Fujimoto, K.; Miyazawa, T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils. J. Nutr. 2000, 130, 3028–3033. [Google Scholar] [PubMed]
- Bolli, R.; Becker, L.; Gross, G.; Mentzer, R.; Balshaw, D.; Lathrop, D.A. Myocardial protection at a crossroads—The need for translation into clinical therapy. Circ. Res. 2004, 95, 125–134. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th ed.; NHMRC: Canberra, Australia, 2004; p. 84. [Google Scholar]
- Lepage, G.; Munoz, G.; Champagne, J.; Roy, C.C. Preparative steps necessary for the accurate measurement of malondialdehyde by high-performance liquid chromatography. Anal. Biochem. 1991, 197, 277–283. [Google Scholar] [CrossRef]
- Yang, C.S.; Jung, L.M. Methodology of plasma retinol, tocopherol and carotenoid assays in cancer prevention studies. J. Nutr. Growth Cancer 1987, 4, 19–27. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Lepage, G.; Roy, C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–121. [Google Scholar] [PubMed]
- Song, J.H.; Miyazawa, T. Enhanced level of n-3 fatty acid in membrane phospholipids induces lipid peroxidation in rats fed dietary docosahexaenoic acid oil. Atherosclerosis 2001, 155, 9–18. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Dotan, Y.; Lichtenberg, D.; Pinchuk, I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog. Lipid Res. 2004, 43, 200–227. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.B.; Tang, X.L.; Guo, Y.; Xuan, Y.T.; Dawn, B.; Bolli, R. Delayed adaptation of the heart to stress—Late preconditioning. Stroke 2004, 35, 2676–2679. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, H.; Fadillioglu, E.; Ozgocmen, S.; Sogut, S.; Ozyurt, B.; Akyol, O.; Ardicoglu, O. Effect of fish oil supplementation on plasma oxidant/antioxidant status in rats. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Fernandes, G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene-expression by omega-3 lipids in murine lupus nephritis. Biochem. Biophys. Res. Commun. 1994, 200, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, J.T.; Chandrasekar, B.; Kim, J.D.; Fernandes, G. Effects of n-3 and n-6 fatty-acids on the activities and expression of hepatic antioxidant enzymes in autoimmune-prone NZBxNZW F1-mice. Lipids 1994, 29, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gutierrez, V.; Vazquez, C.M.; Santa-Maria, C. Liver lipid composition and antioxidant enzyme activities of spontaneously hypertensive rats after ingestion of dietary fats (fish, olive and high-oleic sunflower oils). Biosci. Rep. 2001, 21, 271–285. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.L.; Bridle, T.M.; Abeywardena, M.Y.; Charnock, J.S. Comparative efficacy of n-3 and n-6 polyunsaturated fatty acids in modulating ventricular fibrillation threshold in marmoset monkeys. Am. J. Clin. Nutr. 1993, 58, 666–669. [Google Scholar] [PubMed]
- McLennan, P.L.; Abeywardena, M.Y. Membrane basis for fish oil effects on the heart: Linking natural hibernators to prevention of human sudden cardiac death. J. Membr. Biol. 2005, 206, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.L.; McLennan, P.L.; Owen, A.J.; Theiss, M.L. Low dietary fish oil threshold for myocardial membrane n-3 PUFA enrichment independent of n-6 PUFA intake in rats. J. Lipid Res. 2010, 51, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.; Peoples, G.E.; McLennan, P.L. Muscle fatigue resistance in the rat hindlimb in vivo from low dietary intakes of tuna fish oil that selectively increase phospholipid n-3 docosahexaenoic acid according to muscle fibre type. Br. J. Nutr. 2015, 114, 873–884. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.L.; Owen, A.J.; Slee, E.L.; Theiss, M.L. Myocardial function, ischaemia and n-3 polyunsaturated fatty acids: A membrane basis. J. Cardiovasc. Med. 2007, 8, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Carnes, C.A.; Adamson, P.B.; Vanoli, E.; Schwartz, P.J. Dietary omega-3 fatty acids and susceptibility to ventricular fibrillation lack of protection and a proarrhythmic effect. Circ. Arrhythm. Electrophysiol. 2012, 5, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Tsuduki, T.; Honma, T.; Nakagawa, K.; Ikeda, I.; Miyazawa, T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition 2011, 27, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.L.; Pepe, S. Weighing up fish and omega-3 PUFA advice with accurate, balanced scales: Stringent controls and measures required for clinical trials. Heart Lung Circ. 2015, 24, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. The Second Window of Preconditioning (SWOP) Where Are We Now? Cardiovasc. Drugs Ther. 2010, 24, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.T.; Chen, C.-L.; Ohanyan, V.; Luther, D.J.; Meszaros, J.G.; Chilian, W.M.; Chen, Y.-R. Overexpressing superoxide dismutase 2 induces a supernormal cardiac function by enhancing redox-dependent mitochondrial function and metabolic dilation. J. Mol. Cell. Cardiol. 2015, 88, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Pepe, S.; McLennan, P.L. (n-3) long chain PUFA dose-dependently increase oxygen utilization efficiency and inhibit arrhythmias after saturated fat feeding in rats. J. Nutr. 2007, 137, 2377–2383. [Google Scholar] [PubMed]
- Pepe, S.; McLennan, P.L. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and post-ischemic recovery of contractile function. Circulation 2002, 105, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Vina, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Vina, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Quindry, J.C. Mechanisms of Exercise-Induced Cardioprotection. Physiology 2014, 29, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Oxidative Shielding or Oxidative Stress? J. Pharmacol. Exp. Ther. 2012, 342, 608–618. [Google Scholar] [CrossRef] [PubMed]




| DIET | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid | SF | n-6 PUFA | LCn-3 PUFA | ||||||
| 16:0 | 9.7 | ± | 0.1 | 10.2 | ± | 0.2 | 10.8 | ± | 0.1 |
| 18:0 | 23.7 | ± | 0.2 | 23.8 | ± | 0.1 | 22.4 | ± | 0.2 |
| 18:1n-9 | a 9.5 | ± | 0.1 | b 5.4 | ± | 0.1 | b 4.3 | ± | 0.3 |
| 18:1n-7 | 3.6 | ± | 0.1 | 3.5 | ± | 0.1 | 3.4 | ± | 0.1 |
| Total SFA | 33.80 | ± | 0.13 | 34.70 | ± | 0.80 | 33.70 | ± | 0.40 |
| Total MUFA | a 13.50 | ± | 0.12 | b 8.95 | ± | 0.30 | b 7.75 | ± | 1.10 |
| 18:2n-6 (LA) | b 17.50 | ± | 0.20 | a 18.7 | ± | 0.40 | c 5.60 | ± | 0.03 |
| 20:4n-6 (AA) | a 23.30 | ± | 0.30 | a 23.5 | ± | 0.20 | b 13.30 | ± | 0.15 |
| 22:5n-6 | n.d | a 1.50 | ± | 0.12 | a 1.06 | ± | 0.05 | ||
| 20:5n-3 (EPA) | n.d | n.d | 1.30 | ± | 0.01 | ||||
| 22:5n-3 (DPA) | 1.90 | ± | 0.04 | 1.02 | ± | 0.02 | 1.17 | ± | 0.04 |
| 22:6n-3 (DHA) | b 12.20 | ± | 0.04 | b 10.02 | ± | 0.20 | a 28.30 | ± | 0.04 |
| Total (n-6) PUFA | b 40.80 | ± | 0.20 | a 43.80 | ± | 0.60 | c 20.00 | ± | 0.16 |
| Total (n-3) PUFA | b 14.10 | ± | 0.06 | c 11.00 | ± | 0.20 | a 30.70 | ± | 0.08 |
| Total PUFA | 54.90 | ± | 4.50 | 54.70 | ± | 4.50 | 50.70 | ± | 4.40 |
| UI | b 215.40 | ± | 1.20 | b 215.10 | ± | 0.50 | a 260.58 | ± | 1.20 |
| Peroxidisability Index | b 156.20 | ± | 1.20 | b 149.50 | ± | 1.60 | a 201.10 | ± | 0.70 |
| Dependent Variable | Independent Variable | Association | r2 | p for Slope |
|---|---|---|---|---|
| Ischaemia Protocol | ||||
| Infarct | LPO (ISCH) | positive | 0.337 * | 0.018 |
| Infarct | LPO increase | positive | 0.478 ** | 0.004 |
| Infarct | MDA (ISCH) | positive | 0.356 * | 0.015 |
| Infarct | MDA increase | positive | 0.517 ** | 0.004 |
| Infarct | MnSOD (basal) | negative | 0.851 ** | <0.0001 |
| MDA (ISCH) | LPO (ISCH) | positive | 0.481 ** | 0.006 |
| LPO increase | MnSOD (basal) | negative | 0.397 ** | 0.009 |
| MDA increase | MnSOD (basal) | negative | 0.617 ** | 0.001 |
| IPC + Ischaemia Protocol | ||||
| Infarct | LPO (ISCH) | positive | 0.039 | 0.483 n.s. |
| Infarct | LPO increase | positive | 0.147 | 0.175 n.s. |
| Infarct | MDA (ISCH) | positive | 0.175 | 0.150 n.s. |
| Infarct | MDA increase | positive | 0.009 | 0.728 n.s. |
| Infarct | MnSOD (basal) | negative | 0.058 | 0.335 n.s. |
| MDA (ISCH) | LPO (ISCH) | positive | 0.764 ** | <0.0001 |
| LPO increase | MnSOD (basal) | negative | 0.128 | 0.174 n.s. |
| MDA increase | MnSOD (basal) | negative | 0.293 * | 0.017 |
| Overall | ||||
| Infarct | LPO increase | positive | 0.583 ** | <0.0001 |
| Infarct | MDA increase | positive | 0.475 ** | <0.0001 |
| Infarct | MnSOD (basal) | negative | 0.270 * | 0.0012 |
| MDA (ISCH) | LPO (ISCH) | positive | 0.760 ** | <0.0001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdukeyum, G.G.; Owen, A.J.; Larkin, T.A.; McLennan, P.L. Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. J. Clin. Med. 2016, 5, 32. https://doi.org/10.3390/jcm5030032
Abdukeyum GG, Owen AJ, Larkin TA, McLennan PL. Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. Journal of Clinical Medicine. 2016; 5(3):32. https://doi.org/10.3390/jcm5030032
Chicago/Turabian StyleAbdukeyum, Grace G., Alice J. Owen, Theresa A. Larkin, and Peter L. McLennan. 2016. "Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat" Journal of Clinical Medicine 5, no. 3: 32. https://doi.org/10.3390/jcm5030032
APA StyleAbdukeyum, G. G., Owen, A. J., Larkin, T. A., & McLennan, P. L. (2016). Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. Journal of Clinical Medicine, 5(3), 32. https://doi.org/10.3390/jcm5030032