Galectin-3 Interacts with Vascular Cell Adhesion Molecule-1 to Increase Cardiovascular Mortality in Hemodialysis Patients
Abstract
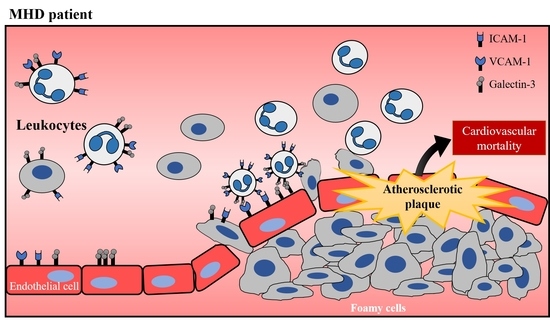
:1. Introduction
2. Methods
2.1. Cohort
2.2. Bio-Demographic and Biochemical Parameters
2.3. Outcomes and Follow-up
2.4. Statistical Methods
3. Results
3.1. Galectin-3
3.2. VCAM-1
3.3. Interaction Analysis between Galectin-3 and VCAM-1 on Mortality Risks
4. Discussion
5. Conclusions
Data Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Alvarez, L.; Ortega, E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediators Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from a to z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chaudhury, P.; Tumlin, J.A.; Koplan, B.A.; Costea, A.I.; Kher, V.; Williamson, D.; Pokhariyal, S.; Charytan, D.M. Primary outcomes of the monitoring in dialysis study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018, 93, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Hsu, S.P.; Pai, M.F.; Yang, J.Y.; Chen, H.Y.; Wu, H.Y.; Peng, Y.S. High soluble vascular cell adhesion molecule-1 concentrations predict long-term mortality in hemodialysis patients. Int. Urol. Nephrol. 2013, 45, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Wang, Z.; Zuberi, R.I.; Sikora, L.; Bahaie, N.S.; Zuraw, B.L.; Liu, F.T.; Sriramarao, P. Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J. Immunol. 2007, 179, 7800–7807. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridonos, M.; McNeill, E.; de Bono, J.P.; Smith, A.; Burnand, K.G.; Channon, K.M.; Greaves, D.R. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Yeh, J.C.; Chiu, Y.L.; Liou, J.C.; Hsiung, J.R.; Tung, T.H. Periodontal pocket depth, hyperglycemia, and progression of chronic kidney disease: A population-based longitudinal study. Am. J. Med. 2017, 130, 61.e1–69.e1. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Feng, Y.F.; Peng, Y.S.; Hsu, S.P.; Pai, M.F.; Chen, H.Y.; Wu, H.Y.; Yang, J.Y. Combined alkaline phosphatase and phosphorus levels as a predictor of mortality in maintenance hemodialysis patients. Medicine (Baltimore) 2014, 93, e106. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Wang, Y.C.; Lee, J.K.; Chang, S.N.; Su, M.Y.; Yeh, H.M.; Su, M.J.; Chen, J.J.; Chiang, F.T.; Hwang, J.J.; et al. Connective tissue growth factor and cardiac diastolic dysfunction: Human data from the Taiwan diastolic heart failure registry and molecular basis by cellular and animal models. Eur. J. Heart Fail. 2014, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Hong, C.Y.; Hou, S.M.; Lin, C.H.; Ong, E.T.; Lee, C.F.; Tsai, C.T.; Lai, L.P. Elevated expression of connective tissue growth factor in human atrial fibrillation and angiotensin ii-treated cardiomyocytes. Circ. J. 2011, 75, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Chen, B.C.; Hsu, M.J.; Tsai, C.T.; Hong, C.Y.; Lin, C.H. Thrombin induced connective tissue growth factor expression in rat vascular smooth muscle cells via the par-1/jnk/ap-1 pathway. Acta Pharmacol. Sin. 2012, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrigal-Matute, J.; Lindholt, J.S.; Fernandez-Garcia, C.E.; Benito-Martin, A.; Burillo, E.; Zalba, G.; Beloqui, O.; Llamas-Granda, P.; Ortiz, A.; Egido, J.; et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Galectin-3, renal function, and clinical outcomes: Results from the luric and 4d studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.; Koenig, W.; Jaensch, A.; Mons, U.; Breitling, L.P.; Scharnagl, H.; Stojakovic, T.; Schunkert, H.; Brenner, H.; Rothenbacher, D. Prognostic utility of galectin-3 for recurrent cardiovascular events during long-term follow-up in patients with stable coronary heart disease: Results of the karola study. Clin. Chem. 2016, 62, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Gi, W.T.; Liao, T.Y.; Lee, M.T.; Lee, S.H.; Hsu, W.T.; Chang, S.S.; Lee, C.C. Using the galectin-3 test to predict mortality in heart failure patients: A systematic review and meta-analysis. Biomark. Med. 2016, 10, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Hogas, S.; Schiller, A.; Voroneanu, L.; Constantinescu, D.; Timar, R.; Cianga, P.; Siriopol, D.; Bob, F.; Cianga, C.; Onofriescu, M.; et al. Predictive value for galectin 3 and cardiotrophin 1 in hemodialysis patients. Angiology 2016, 67, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; O'Donoghue, M.L. Galectin-3 in cardiovascular disease: A possible window into early myocardial fibrosis. J. Am. Coll. Cardiol. 2012, 60, 1257–1258. [Google Scholar] [CrossRef] [PubMed]



| Variables | Overall (n = 86) | Survivors (n = 51) | Deceased (n = 35) |
|---|---|---|---|
| Age (years) | 59.9 ± 14.0 | 52.6 ± 13.1 | 67.5 ± 8.6 |
| Male, n (%) | 38 (44.2) | 23 (45.1) | 15 (42.9) |
| Diabetes mellitus, n (%) | 35 (40.7) | 19 (37.3) | 16 (45.7) |
| Hypertension, n (%) | 39 (45.3) | 22 (43.1) | 17 (48.6) |
| Smoking, n (%) | 19 (22.1) | 5 (9.8) | 14 (40.0) |
| Systolic blood pressure (mmHg) | 135 ± 25 | 137 ± 25 | 129 ± 22 |
| Diastolic blood pressure (mmHg) | 76 ± 12 | 78 ± 13 | 72 ± 11 |
| Hemodialysis vintage (months) | 61.2 ± 53.2 | 58.9 ± 51.6 | 64.5 ± 56.1 |
| Alanine aminotransferase (IU/L) | 13.3 ± 10.2 | 15.5 ± 11.9 | 11.1 ± 6.2 |
| Albumin (g/dL) | 3.9 ± 0.5 | 4.0 ± 0.3 | 3.6 ± 0.5 |
| Total cholesterol (mg/dL) | 194.0 ± 49.3 | 195.2 ± 51.3 | 192.3 ± 47.1 |
| Triglyceride (mg/dL) | 210.0 ± 179.0 | 201.8 ± 170.5 | 212.0 ± 193.1 |
| Creatinine (mg/dL) | 10.3 ± 1.7 | 10.6 ± 1.8 | 9.9 ± 1.8 |
| Uric acid (mg/dL) | 7.3 ± 1.3 | 7.5 ± 1.4 | 7.1 ± 1.1 |
| Normalized protein catabolic rate (g/kg/day) | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.9 ± 0.3 |
| Potassium (mmol/L) | 4.6 ± 0.9 | 4.6 ± 0.8 | 4.6 ± 1.0 |
| Adjusted calcium (mg/dL) | 9.2 ± 0.7 | 9.3 ± 0.7 | 9.1 ± 0.7 |
| Phosphate (mg/dL) | 4.4 ± 1.6 | 4.6 ± 1.7 | 4.3 ± 1.3 |
| Intact parathyroid hormone (pg/mL) | 150.9 ± 192.7 | 235.0 ± 32.9 | 98.3 ± 16.6 |
| Iron (μg/dL) | 79.4 ± 33.0 | 81.5 ± 34.7 | 74.4 ± 29.4 |
| Total iron binding capacity (μg/dL) | 230.0 ± 39.7 | 236.9 ± 40.6 | 226.1 ± 37.3 |
| Ferritin (ng/mL) | 623.0 ± 317.7 | 579.5 ± 286.7 | 665.1 ± 353.1 |
| Hemoglobin (g/dL) | 11.0 ± 1.0 | 10.7 ± 1.3 | 10.4 ± 1.5 |
| C-reactive protein (mg/dL) | 0.7 ± 1.0 | 0.6 ± 0.5 | 1.1 ± 1.2 |
| Galectin-3 (ng/mL) | 29.5 ± 10.3 | 25.5 ± 9.6 | 35.2 ± 8.6 |
| Vascular cell adhesion molecule 1 (ng/mL) | 1546.9 ± 331.8 | 1458.6 ± 303.7 | 1675.9 ± 332.8 |
| Galectin-3 | VCAM-1 | |
|---|---|---|
| Galectin-3 | 1.00 | 0.49 ** |
| Vascular cell adhesion molecule 1 | 0.49 ** | 1.00 |
| Age | 0.06 | 0.05 |
| Hemodialysis vintage | 0.07 | 0.22 * |
| Normalized protein catabolic rate | −0.31 ** | −0.212 |
| Albumin | −0.13 | −0.21 * |
| C-reactive protein | 0.32 ** | 0.24 * |
| Creatinine | −0.14 | −0.12 |
| Total cholesterol | −0.18 | −0.19 |
| Triglyceride | −0.08 | −0.17 |
| Low-density lipoprotein | −0.16 | 0.17 |
| Uric acid | 0.03 | −0.08 |
| Potassium | −0.10 | −0.15 |
| Glucose | 0.03 | −0.08 |
| Adjusted calcium | 0.02 | −0.15 |
| Phosphate | −0.17 | −0.01 |
| Hemoglobin | −0.12 | −0.25 * |
| Smoking | 0.18 | 0.26 * |
| Cox Univariate | Cox Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Galectin-3 | 1.058 (1.031–1.085) | p < 0.01 | 1.038 (1.001–1.077) | p < 0.05 |
| VCAM-1 | 1.002 (1.001–1.003) | p < 0.01 | 1.002 (1.001–1.003) | p < 0.01 |
| Age | 1.062 (1.031–1.094) | p < 0.01 | 1.068 (1.031–1.106) | p < 0.01 |
| CRP | 1.997 (1.487–2.681) | p < 0.01 | 1.686 (1.127–2.522) | p < 0.05 |
| Albumin | 0.423 (0.217–0.823) | p < 0.05 | 1.701 (0.627–4.611) | p > 0.05 |
| nPCR | 0.081 (0.022–0.295) | p < 0.01 | 0.158 (0.036–0.698) | p < 0.05 |
| Smoking | 2.813 (0.813–9.727) | p > 0.05 | 2.360 (0.860–6.475) | p > 0.05 |
| Cox Univariate | Cox Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Galectin-3 | 1.061 (1.028–1.096) | p < 0.01 | 1.043 (0.993–1.096) | p > 0.05 |
| VCAM-1 | 1.002 (1.001–1.003) | p < 0.01 | 1.002 (1.001–1.003) | p < 0.05 |
| Age | 1.116 (1.056–1.180) | p < 0.01 | 1.126 (1.059–1.196) | p < 0.01 |
| CRP | 2.200 (1.512–3.202) | p < 0.01 | 2.612 (1.324–5.151) | p < 0.01 |
| Albumin | 0.480 (0.199–1.159) | p > 0.05 | 4.599 (0.878–14.10) | p > 0.05 |
| nPCR | 0.171 (0.035–0.841) | p < 0.05 | 0.628 (0.088–4.469) | p > 0.05 |
| Smoking | 3.383 (1.386–8.261) | p < 0.01 | 1.559 (0.346–7.035) | p > 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, W.-C.; Choy, C.-S.; Lin, W.-N.; Chang, S.-W.; Liou, J.-C.; Tung, T.-H.; Hsieh, C.-Y.; Chang, J.-F. Galectin-3 Interacts with Vascular Cell Adhesion Molecule-1 to Increase Cardiovascular Mortality in Hemodialysis Patients. J. Clin. Med. 2018, 7, 300. https://doi.org/10.3390/jcm7100300
Ko W-C, Choy C-S, Lin W-N, Chang S-W, Liou J-C, Tung T-H, Hsieh C-Y, Chang J-F. Galectin-3 Interacts with Vascular Cell Adhesion Molecule-1 to Increase Cardiovascular Mortality in Hemodialysis Patients. Journal of Clinical Medicine. 2018; 7(10):300. https://doi.org/10.3390/jcm7100300
Chicago/Turabian StyleKo, Wen-Chin, Cheuk-Sing Choy, Wei-Ning Lin, Shu-Wei Chang, Jian-Chiun Liou, Tao-Hsin Tung, Chih-Yu Hsieh, and Jia-Feng Chang. 2018. "Galectin-3 Interacts with Vascular Cell Adhesion Molecule-1 to Increase Cardiovascular Mortality in Hemodialysis Patients" Journal of Clinical Medicine 7, no. 10: 300. https://doi.org/10.3390/jcm7100300
APA StyleKo, W. -C., Choy, C. -S., Lin, W. -N., Chang, S. -W., Liou, J. -C., Tung, T. -H., Hsieh, C. -Y., & Chang, J. -F. (2018). Galectin-3 Interacts with Vascular Cell Adhesion Molecule-1 to Increase Cardiovascular Mortality in Hemodialysis Patients. Journal of Clinical Medicine, 7(10), 300. https://doi.org/10.3390/jcm7100300