Epigenetic Changes at the Birc5 Promoter Induced by YM155 in Synovial Sarcoma
Abstract
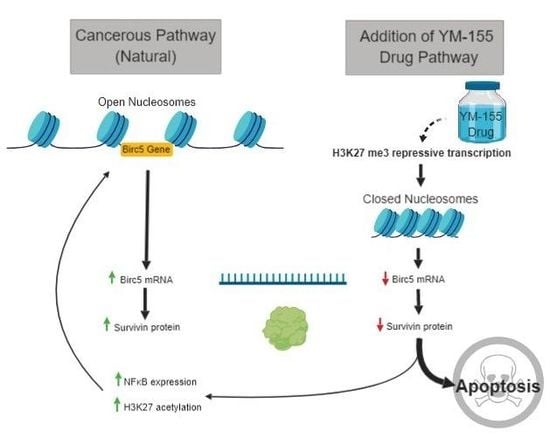
:1. Introduction
2. Experimental Section
2.1. Human and Mouse Expression Databases
2.2. Mice
2.3. Cell Lines
2.4. YM155 Mouse Treatments
2.5. MTT Viability Assay and caspase 3/7 and caspase 8 Enzymatic Activity
2.6. Immunohistochemistry and Immunoblotting
2.7. qPCR Birc5 Detection
2.8. Chromatin Immunoprecipitation–Quantitative PCR
2.9. Statistical Methods
3. Results
3.1. Clinical Trials Data on YM155
3.2. Synovial Sarcoma Exhibits Similar BIRC5 Expression Levels to Other Cancers
3.3. Birc5/Survivin Expression in a Mouse Model of Synovial Sarcoma
3.3.1. YM155 Inhibition of Human Synovial Sarcoma Cells in Vitro
3.3.2. YM155 Inhibition of Murine Synovial Sarcomas in Vivo
3.3.3. The Effects of YM155 Inhibition in Murine Synovial Sarcoma on Birc5/Survivin
3.4. Establishing an in Vitro Model to Determine the Mechanism of Action of YM155
3.5. Histone lysine Modifications Suggest an Epigenetic Mechanism of Action of YM155
3.6. The Transcription Factor NFκB Increases upon YM155 Treatment, but Fails to Activate Birc5
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



References
- Rajwanshi, A.; Srinivas, R.; Upasana, G. Malignant small round cell tumors. J. Cytol. 2009, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Fisher, C. Synovial sarcoma: Defining features and diagnostic evolution. Ann. Diagn. Pathol. 2014, 18, 369–380. [Google Scholar] [CrossRef]
- Ladanyi, M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene 2001, 20, 5755–5762. [Google Scholar] [CrossRef] [Green Version]
- Kadoch, C.; Crabtree, G.R. Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell 2013, 153, 71–85. [Google Scholar] [CrossRef]
- Krieg, A.H.; Hefti, F.; Speth, B.M.; Jundt, G.; Guillou, L.; Exner, U.G.; von Hochstetter, A.R.; Cserhati, M.D.; Fuchs, B.; Mouhsine, E.; et al. Synovial sarcomas usually metastasize after >5 years: A multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann. Oncol. 2011, 22, 458–467. [Google Scholar] [CrossRef]
- Vlenterie, M.; Vlenterie, M.; Litière, S.; Rizzo, E.; Marréaud, S.; Judson, I.; Gelderblom, H.; Le Cesne, A.; Wardelmann, E.; Messiou, C.; et al. Outcome of chemotherapy in advanced synovial sarcoma patients: Review of 15 clinical trials from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group; setting a new landmark for studies in this entity. Eur. J. Cancer 2016, 58, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Eilber, F.C.; Brennan, M.F.; Eilber, F.R.; Eckardt, J.J.; Grobmyer, S.R.; Riedel, E.; Forscher, C.; Maki, R.G.; Singer, S. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann. Surg. 2007, 246, 105–113. [Google Scholar] [CrossRef]
- Karavasilis, V.; Seddon, B.M.; Ashley, S.; Al-Muderis, O.; Fisher, C.; Judson, I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: Retrospective analysis and identification of prognostic factors in 488 patients. Cancer 2008, 112, 1585–1591. [Google Scholar] [CrossRef]
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [Green Version]
- Yamashita, S.; Masuda, Y.; Kurizaki, T.; Haga, Y.; Murayama, T.; Ikei, S.; Kamei, M.; Takeno, S.; Kawahara, K. Survivin expression predicts early recurrence in early-stage breast cancer. Anticancer Res. 2007, 27, 2803–2808. [Google Scholar] [PubMed]
- Taubert, H.; Heidenreich, C.; Holzhausen, H.J.; Schulz, A.; Bache, M.; Kappler, M.; Eckert, A.W.; Würl, P.; Melcher, I.; Hauptmann, K.; et al. Expression of survivin detected by immunohistochemistry in the cytoplasm and in the nucleus is associated with prognosis of leiomyosarcoma and synovial sarcoma patients. BMC Cancer 2010, 10, 65. [Google Scholar] [CrossRef]
- Altieri, D.C. Survivin and IAP proteins in cell-death mechanisms. Biochem. J. 2010, 430, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ling, X.; Haller, A.; Nakahara, T.; Yamanaka, K.; Kita, A.; Koutoku, H.; Takeuchi, M.; Brattain, M.G.; Li, F. Suppression of survivin promoter activity by YM155 involves disruption of Sp1-DNA interaction in the survivin core promoter. Int. J. Biochem. Mol. Biol. 2012, 3, 179–197. [Google Scholar] [PubMed]
- Yamauchi, T.; Nakamura, N.; Hiramoto, M.; Yuri, M.; Yokota, H.; Naitou, M.; Takeuchi, M.; Yamanaka, K.; Kita, A.; Nakahara, T.; et al. Sepantronium bromide (YM155) induces disruption of the ILF3/p54(nrb) complex, which is required for survivin expression. Biochem. Biophys. Res. Commun. 2012, 425, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Hennig, D.; Schäfer, C.; Wirth, M.; Marx, C.; Heinzel, T.; Schneider, G.; Krämer, O.H. Survivin and YM155: How faithful is the liaison? Biochim. Biophys. Acta 2014, 1845, 202–220. [Google Scholar] [CrossRef]
- Tang, H.; Shao, H.; Yu, C.; Hou, J. Mcl-1 downregulation by YM155 contributes to its synergistic anti-tumor activities with ABT-263. Biochem. Pharmacol. 2011, 82, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.S.; Yang, S.J.; Kim, S.M.; Jung, K.A.; Moon, J.H.; Shin, J.S.; Yoon, D.H.; Hong, Y.S.; Ryu, M.H.; Lee, J.L.; et al. YM155 induces EGFR suppression in pancreatic cancer cells. PLoS ONE 2012, 7, e38625. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.C.; Chen, S.H.; Yang, S.H.; Cheng, C.C.; Chiu, T.H.; Huang, Y.T. Novel survivin inhibitor YM155 elicits cytotoxicity in glioblastoma cell lines with normal or deficiency DNA-dependent protein kinase activity. Pediatr. Neonatol. 2012, 53, 199–204. [Google Scholar] [CrossRef]
- Shaul, Y.D.; Yuan, B.; Thiru, P.; Nutter-Upham, A.; McCallum, S.; Lanzkron, C.; Bell, G.W.; Sabatini, D.M. MERAV: A tool for comparing gene expression across human tissues and cell types. Nucleic Acids Res. 2016, 44, D560–D566. [Google Scholar] [CrossRef]
- Barrott, J.J.; Kafchinski, L.A.; Jin, H.; Potter, J.W.; Kannan, S.D.; Kennedy, R.; Mosbruger, T.; Wang, W.L.; Tsai, J.W.; Araujo, D.M.; et al. Modeling synovial sarcoma metastasis in the mouse: PI3’-lipid signaling and inflammation. J. Exp. Med. 2016, 213, 2989–3005. [Google Scholar] [CrossRef] [PubMed]
- Barrott, J.J.; Illum, B.E.; Jin, H.; Zhu, J.F.; Mosbruger, T.; Monument, M.J.; Smith-Fry, K.; Cable, M.G.; Wang, Y.; Grossmann, A.H.; et al. beta-catenin stabilization enhances SS18-SSX2-driven synovial sarcomagenesis and blocks the mesenchymal to epithelial transition. Oncotarget 2015, 6, 22758–22766. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Thomas, A.; Rajan, A.; Chun, G.; Lopez-Chavez, A.; Szabo, E.; Spencer, S.; Carter, C.A.; Guha, U.; Khozin, S.; et al. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2013, 24, 2601–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, M.R.; Gladkov, O.A.; Gartner, E.; Vladimirov, V.; Crown, J.; Steinberg, J.; Jie, F.; Keating, A. Phase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancer. Breast Cancer Res. Treat. 2015, 149, 171–179. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Mita, A.; Lewis, L.D.; Garrett, C.R.; Till, E.; Daud, A.I.; Patnaik, A.; Papadopoulos, K.; Takimoto, C.; Bartels, P.; et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J. Clin. Oncol. 2008, 26, 5198–5203. [Google Scholar] [CrossRef]
- Heintzman, N.D.; Stuart, R.K.; Hon, G.; Fu, Y.; Ching, C.W.; Hawkins, R.D.; Barrera, L.O.; Van Calcar, S.; Qu, C.; Ching, K.A.; et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007, 39, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.; Vethantham, V.; Bowman, C.; Rudnicki, M.; Dynlacht, B.D. Genome-wide identification of enhancers in skeletal muscle: The role of MyoD1. Genes Dev. 2012, 26, 2763–2779. [Google Scholar] [CrossRef]
- Cheng, J.; Blum, R.; Bowman, C.; Hu, D.; Shilatifard, A.; Shen, S.; Dynlacht, B.D. A role for H3K4 monomethylation in gene repression and partitioning of chromatin readers. Mol. Cell 2014, 53, 979–992. [Google Scholar] [CrossRef]
- Wang, K.; Brems, J.J.; Gamelli, R.L.; Holterman, A.X. Survivin signaling is regulated through nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim. Biophys. Acta 2010, 1803, 1368–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, A.; Yamauchi, N.; Shibahara, J.; Kimura, H.; Morikawa, T.; Ishikawa, S.; Nagae, G.; Nishi, A.; Sakamoto, Y.; Kokudo, N.; et al. Concurrent activation of acetylation and tri-methylation of H3K27 in a subset of hepatocellular carcinoma with aggressive behavior. PLoS ONE 2014, 9, e91330. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Barrott, J.J.; Cable, M.G.; Monument, M.J.; Lerman, D.M.; Smith-Fry, K.; Nollner, D.; Jones, K.B. The Impact of Microenvironment on the Synovial Sarcoma Transcriptome. Cancer Microenviron. 2017, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, M.; Shiraki, K.; Inoue, H.; Okano, H.; Ito, T.; Yamanaka, T.; Sugimoto, K.; Sakai, T.; Ohmori, S.; Murata, K.; et al. Expression of survivin during liver regeneration. Biochem. Biophys. Res. Commun. 2002, 297, 59–64. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Yamauchi, T.; Hiramoto, M.; Yuri, M.; Naito, M.; Takeuchi, M.; Yamanaka, K.; Kita, A.; Nakahara, T.; Kinoyama, I.; et al. Interleukin enhancer-binding factor 3/NF110 is a target of YM155, a suppressant of survivin. Mol. Cell. Proteom. 2012, 11, M111.013243. [Google Scholar] [CrossRef] [PubMed]









| Study Identifier | Number of Patients | Phase | Disease | Date Ended |
|---|---|---|---|---|
| NCT01023386 | 6 | phase 1 | cancer | 2010 |
| NCT00818480 | 10 | phase 2 | prostate cancer, melanoma, non-Hodgkin′s Lymphoma | 2012 |
| NCT01007292 | 43 | phase 2 | non-Hodgkin′s Lymphoma | 2015 |
| NCT01009775 | 64 | phase 2 | melanoma | 2012 |
| NCT01038804 | 101 | phase 2 | breast cancer | 2013 |
| NCT00498914 | 41 | phase 2 | lymphoma | 2009 |
| NCT01100931 | 42 | phase 1/2 | NSCLC 2, solid tumors | 2015 |
| NCT00514267 | 32 | phase 1/2 | prostate cancer, tumors | 2015 |
| NCT00281541 | 29 | phase 2 | melanoma | 2012 |
| NCT00328588 | 37 | phase 2 | NSCLC 2 | 2008 |
| NCT00257478 | NA 1 | phase 2 | prostate cancer | 2007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mika, A.; Luelling, S.E.; Pavek, A.; Nartker, C.; Heyneman, A.L.; Jones, K.B.; Barrott, J.J. Epigenetic Changes at the Birc5 Promoter Induced by YM155 in Synovial Sarcoma. J. Clin. Med. 2019, 8, 408. https://doi.org/10.3390/jcm8030408
Mika A, Luelling SE, Pavek A, Nartker C, Heyneman AL, Jones KB, Barrott JJ. Epigenetic Changes at the Birc5 Promoter Induced by YM155 in Synovial Sarcoma. Journal of Clinical Medicine. 2019; 8(3):408. https://doi.org/10.3390/jcm8030408
Chicago/Turabian StyleMika, Aleksander, Sarah E. Luelling, Adriene Pavek, Christopher Nartker, Alexandra L. Heyneman, Kevin B. Jones, and Jared J. Barrott. 2019. "Epigenetic Changes at the Birc5 Promoter Induced by YM155 in Synovial Sarcoma" Journal of Clinical Medicine 8, no. 3: 408. https://doi.org/10.3390/jcm8030408
APA StyleMika, A., Luelling, S. E., Pavek, A., Nartker, C., Heyneman, A. L., Jones, K. B., & Barrott, J. J. (2019). Epigenetic Changes at the Birc5 Promoter Induced by YM155 in Synovial Sarcoma. Journal of Clinical Medicine, 8(3), 408. https://doi.org/10.3390/jcm8030408