Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration
Abstract
:1. Introduction
2. Experimental Section
2.1. Isolation and Culture of Human Adipose-Derived Mesenchymal Stem Cells (hASCs)
2.2. Proliferation Rate Assay
2.3. Extracellular Oxidative Stress Evaluation
2.4. Determination of Cellular Senescence
2.5. Cell Morphology Evaluation (SEM Analysis)
2.6. Fluorescent Microscopy
2.7. Quantitative Cell Analysis Using a Flow Cytometry-Based System
2.8. ELISA Assay (VEGF, CXCL12/SDF-1, Adiponectin, and Leptin)
2.9. Analysis of mRNA and microRNA Expression (RT-qPCR)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Type 2 Diabetes Diminishes ASC Proliferation and Increases Apoptosis
3.2. Cellular Senescence and Extracellular Oxidative Stress in ASCs are Associated with Type 2 Diabetes
3.3. Comparison of the Mitochondrial Dynamics and Mitochondrial Membrane Potential
3.4. Expression of mRNA and miRNA Associated with Cell Proliferation and Insulin Resistance
3.5. Alternations in Selected miRNA and Protein Levels in ASCs Derived from T2D Patients Exhibit their Impairment of Proliferation Activity, Regenerative Potential, and Insulin Sensitivity
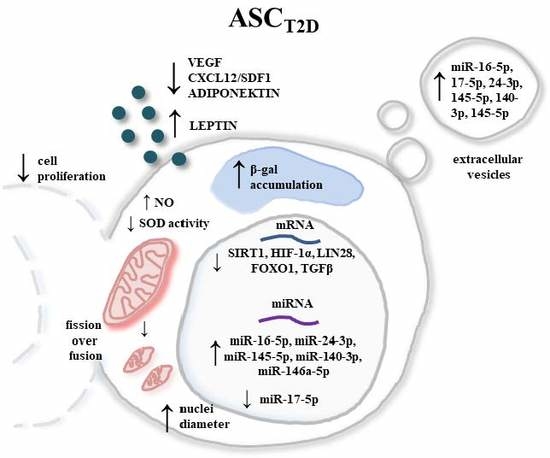
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; Roglic, G., Ed.; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-156525-7. [Google Scholar]
- What is Diabetes? | NIDDK. Available online: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes (accessed on 2 March 2019).
- Cusi, K. The Role of Adipose Tissue and Lipotoxicity in the Pathogenesis of Type 2 Diabetes. Curr. Diabetes Rep. 2010, 10, 306–315. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spence, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation Versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef]
- Grzesiak, J.; Krzysztof, M.; Karol, W.; Joanna, C. Isolation and Morphological Characterisation of Ovine Adipose-Derived Mesenchymal Stem Cells in Culture. Int. J. Stem Cells 2011, 4, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor Age Negatively Impacts Adipose Tissue-Derived Mesenchymal Stem Cell Expansion and Differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.; Fiorina, P.; Adra, C.N.; Atkinson, M.; Sayegh, M.H. Immunomodulation by Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Type 1 Diabetes. Diabetes 2008, 57, 1759–1767. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Med. Inflamm. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Marędziak, M.; Marycz, K.; Lewandowski, D.; Siudzińska, A.; Śmieszek, A. Static magnetic Field Enhances Synthesis and Secretion of Membrane-Derived Microvesicles (MVs) Rich in VEGF and BMP-2 in Equine Adipose-Derived Stromal Cells (EqASCs)—A New Approach in Veterinary Regenerative Medicine. In Vitro Cell Dev. Biol. Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient Scalable Production of Therapeutic Microvesicles Derived from Human Mesenchymal Stem Cells. Sci. Rep. 2018, 8, 1171. [Google Scholar] [CrossRef]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF Secreted by Mesenchymal Stem Cells Mediates the Differentiation of Endothelial Progenitor Cells into Endothelial Cells Via Paracrine Mechanisms. Mol. Med. Rep. 2018, 17, 1667–1675. [Google Scholar] [CrossRef]
- Bonventre, J.V. Microvesicles from Mesenchymal Stromal Cells Protect Against Acute Kidney Injury. J. Am. Soc. Nephrol. 2009, 20, 927–928. [Google Scholar] [CrossRef]
- De Almeida, D.C.; Bassi, Ê.J.; Azevedo, H.; Anderson, L.; Origassa, C.S.T.; Cenedeze, M.A.; De Andrade-Oliveira, V.; Felizardo, R.J.F.; Da Silva, R.C.; Hiyane, M.I.; et al. A Regulatory miRNA–mRNA Network Is Associated with Tissue Repair Induced by Mesenchymal Stromal Cells in Acute Kidney Injury. Front. Immunol. 2017, 7, 645. [Google Scholar] [CrossRef]
- De Candia, P.; Spinetti, G.; Specchia, C.; Sangalli, E.; La Sala, L.; Uccellatore, A.; Lupini, S.; Genovese, S.; Matarese, G.; Ceriello, A. A Unique Plasma MicroRNA Profile Defines Type 2 Diabetes Progression. PLoS ONE 2017, 12, e0188980. [Google Scholar] [CrossRef]
- Ferrer-Lorente, R.; Bejar, M.T.; Tous, M.; Vilahur, G.; Badimon, L. Systems Biology Approach to Identify Alterations in the Stem Cell Reservoir of Subcutaneous Adipose Tissue in a Rat Model of Diabetes: Effects on Differentiation Potential and Function. Diabetologia 2014, 57, 246–256. [Google Scholar] [CrossRef]
- Qian, X.; Villa-Diaz, L.G.; Krebsbach, P.H. Advances in Culture and Manipulation of Human Pluripotent Stem Cells. J. Dent. Res. 2013, 92, 956–962. [Google Scholar] [CrossRef]
- Alicka, M.; Marycz, K. The Effect of Chronic Inflammation and Oxidative and Endoplasmic Reticulum Stress in the Course of Metabolic Syndrome and Its Therapy. Stem Cells Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Carrier, A. Metabolic Syndrome and Oxidative Stress: A Complex Relationship. Antioxid. Redox Sign. 2017, 26, 429–431. [Google Scholar] [CrossRef]
- Mahjoub, S.; Masrour-Roudsari, J. Role of Oxidative Stress in Pathogenesis of Metabolic Syndrome. Casp. J. Intern. Med. 2012, 3, 386–396. [Google Scholar]
- Park, S.G.; Kim, J.-H.; Xia, Y.; Sung, J.-H. Generation of Reactive Oxygen Species in Adipose-Derived Stem Cells: Friend or Foe? Expert Opin. Ther. Targets 2011, 15, 1297–1306. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca(2+) Homeostasis and Endoplasmic Reticulum (ER) Stress: An Integrated View of Calcium Signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Jha, S.K.; Jha, N.K.; Kumar, D.; Ambasta, R.K.; Kumar, P. Linking Mitochondrial Dysfunction, Metabolic Syndrome and Stress Signaling in Neurodegeneration. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1132–1146. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Metabolic Regulation of Mitochondrial Dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef]
- Del Campo, A.; Bustos, C.; Mascayano, C.; Acuña-Castillo, C.; Troncoso, R.; Rojo, L.E. Metabolic Syndrome and Antipsychotics: The Role of Mitochondrial Fission/Fusion Imbalance. Front. Endocrinol. (Lausanne) 2018, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Kornicka, K.; Marycz, K.; Tomaszewski, K.A.; Marędziak, M.; Śmieszek, A. The Effect of Age on Osteogenic and Adipogenic Differentiation Potential of Human Adipose Derived Stromal Stem Cells (hASCs) and the Impact of Stress Factors in the Course of the Differentiation Process. Oxid. Med. Cell Longev. 2015, 2015, 309169. [Google Scholar] [CrossRef]
- Ye, X.; Liao, C.; Liu, G.; Xu, Y.; Tan, J.; Song, Z. Age-Related Changes in the Regenerative Potential of Adipose-Derived Stem Cells Isolated from the Prominent Fat Pads in Human Lower Eyelids. PLoS ONE 2016, 11, e0166590. [Google Scholar] [CrossRef]
- Marycz, K.; Weiss, C.; Śmieszek, A.; Kornicka, K. Evaluation of Oxidative Stress and Mitophagy during Adipogenic Differentiation of Adipose-Derived Stem Cells Isolated from Equine Metabolic Syndrome (EMS) Horses. Stem Cells Int. 2018, 2018, 5340756. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxid. Med. Cell Longev. 2016, 2016, 4710326. [Google Scholar] [CrossRef]
- Jumabay, M.; Moon, J.H.; Yeerna, H.; Boström, K.I. Effect of Diabetes Mellitus on Adipocyte-Derived Stem Cells in Rat. J. Cell. Physiol. 2015, 230, 2821–2828. [Google Scholar] [CrossRef]
- Roth, V. Doubling Time Computing 2006. Available online: http://www.doubling-time.com/compute.php (accessed on 2 March 2019).
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Urruticoechea, A.; Smith, I.E.; Dowsett, M. Proliferation Marker Ki-67 in Early Breast Cancer. J. Clin. Oncol. 2005, 23, 7212–7220. [Google Scholar] [CrossRef] [PubMed]
- Inwald, E.C.; Klinkhammer-Schalke, M.; Hofstädter, F.; Zeman, F.; Koller, M.; Gerstenhauer, M.; Ortmann, O. Ki-67 is a Prognostic Parameter in Breast Cancer Patients: Results of a Large Population-Based Cohort of a Cancer Registry. Breast Cancer Res. Treat. 2013, 139, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Grzesiak, J.; Śmieszek, A.; Szłapka, J. Macroautophagy and Selective Mitophagy Ameliorate Chondrogenic Differentiation Potential in Adipose Stem Cells of Equine Metabolic Syndrome: New Findings in the Field of Progenitor Cells Differentiation. Oxid. Med. Cell Longev. 2016, 2016, 3718468. [Google Scholar] [CrossRef]
- Révész, D.; Milaneschi, Y.; Verhoeven, J.E.; Lin, J.; Penninx, B.W.J.H. Longitudinal Associations Between Metabolic Syndrome Components and Telomere Shortening. J. Clin. Endocrinol. Metab. 2015, 100, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Biran, A.; Zada, L.; Abou Karam, P.; Vadai, E.; Roitman, L.; Ovadya, Y.; Porat, Z.; Krizhanovsky, V. Quantitative Identification of Senescent Cells in Aging and Disease. Aging Cell 2017, 16, 661–671. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and Telomerase in Stem Cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef]
- Kirchner, H.; Shaheen, F.; Kalscheuer, H.; Schmid, S.M.; Oster, H.; Lehnert, H. The Telomeric Complex and Metabolic Disease. Genes (Basel) 2017, 8, 176. [Google Scholar] [CrossRef]
- Zhao, H.; Darzynkiewicz, Z. Biomarkers of Cell Senescence Assessed by Imaging Cytometry. In Cell Senescence; Galluzzi, L., Vitale, I., Kepp, O., Kroemer, G., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 965, pp. 83–92. ISBN 978-1-62703-238-4. [Google Scholar]
- Yoon, K.B.; Park, K.R.; Kim, S.Y.; Han, S.-Y. Induction of Nuclear Enlargement and Senescence by Sirtuin Inhibitors in Glioblastoma Cells. Immune Netw. 2016, 16, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Ozanne, S.E.; Hales, C.N. Methods of Cellular Senescence Induction Using Oxidative Stress. Methods Mol. Biol. 2007, 371, 179–189. [Google Scholar]
- Choo, K.B.; Tai, L.; Hymavathee, K.S.; Wong, C.Y.; Nguyen, P.N.N.; Huang, C.-J.; Cheong, S.K.; Kamarul, T. Oxidative Stress-Induced Premature Senescence in Wharton’s Jelly-Derived Mesenchymal Stem Cells. Int. J. Med. Sci. 2014, 11, 1201–1207. [Google Scholar] [CrossRef]
- Nawrocka, D.; Kornicka, K.; Śmieszek, A.; Marycz, K. Spirulina Platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Mar. Drugs 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [Green Version]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial Dynamics: Overview of Molecular Mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.A.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and Selective Fusion Govern Mitochondrial Segregation and Elimination by Autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Wang, Q.; Frolova, A.I.; Purcell, S.; Adastra, K.; Schoeller, E.; Chi, M.M.; Schedl, T.; Moley, K.H. Mitochondrial Dysfunction and Apoptosis in Cumulus Cells of Type I Diabetic Mice. PLoS ONE 2010, 5, e15901. [Google Scholar] [CrossRef]
- Kitada, M.; Koya, D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Simic, P.; Zainabadi, K.; Bell, E.; Sykes, D.B.; Saez, B.; Lotinun, S.; Baron, R.; Scadden, D.; Schipani, E.; Guarente, L. SIRT1 Regulates Differentiation of Mesenchymal Stem Cells by Deacetylating β-Catenin. EMBO Mol. Med. 2013, 5, 430–440. [Google Scholar] [CrossRef]
- Lv, B.; Li, F.; Fang, J.; Xu, L.; Sun, C.; Han, J.; Hua, T.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia Inducible Factor 1α Promotes Survival of Mesenchymal Stem Cells Under Hypoxia. Am. J. Transl. Res. 2017, 9, 1521–1529. [Google Scholar] [PubMed]
- Jiang, C.; Qu, A.; Matsubara, T.; Chanturiya, T.; Jou, W.; Gavrilova, O.; Shah, Y.M.; Gonzalez, F.J. Disruption of Hypoxia-Inducible Factor 1 in Adipocytes Improves Insulin Sensitivity and Decreases Adiposity in High-Fat Diet–Fed Mice. Diabetes 2011, 60, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Liu, H.; Lv, X.; Liu, Y.; Wang, X.; Zhang, M.; Zhang, X.; Li, Y.; Lou, Q.; Li, S.; et al. MicroRNA-16-5p Overexpression Suppresses Proliferation and Invasion as Well as Triggers Apoptosis by Targeting VEGFA Expression in Breast Carcinoma. Oncotarget 2017, 8, 72400–72410. [Google Scholar] [CrossRef]
- He, Y.; Ding, Y.; Liang, B.; Lin, J.; Kim, T.-K.; Yu, H.; Hang, H.; Wang, K. A Systematic Study of Dysregulated MicroRNA in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2017, 18, 456. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fu, N.; Yang, P. MiR-17 Downregulation by High Glucose Stabilizes Thioredoxin-Interacting Protein and Removes Thioredoxin Inhibition on ASK1 Leading to Apoptosis. Toxicol. Sci. 2016, 150, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Takahashi, T.; Masuda, H.; Kalka, C.; Chen, D.; Iwaguro, H.; Inai, Y.; Silver, M.; Isner, J.M. VEGF Contributes to Postnatal Neovascularization by Mobilizing Bone Marrow-Derived Endothelial Progenitor Cells. EMBO J. 1999, 18, 3964–3972. [Google Scholar] [CrossRef]
- Hu, C.; Yong, X.; Li, C.; Lü, M.; Liu, D.; Chen, L.; Hu, J.; Teng, M.; Zhang, D.; Fan, Y.; et al. CXCL12/CXCR4 Axis Promotes Mesenchymal Stem Cell Mobilization to Burn Wounds and Contributes to Wound Repair. J. Surg. Res. 2013, 183, 427–434. [Google Scholar] [CrossRef]
- Kočí, Z.; Turnovcová, K.; Dubský, M.; Baranovičová, L.; Holáň, V.; Chudíčková, M.; Syková, E.; Kubinová, Š. Characterization of Human Adipose Tissue-Derived Stromal Cells Isolated from Diabetic Patient’s Distal Limbs with Critical Ischemia. Cell Biochem. Funct. 2014, 32, 597–604. [Google Scholar] [CrossRef]





| Gene | Primer Sequence (Forward/Reverse) | Amplicon Size (bp) | Accession Number |
|---|---|---|---|
| SIRT-1 | ACAGGTTGCGGGAATCCAAA/ | 155 | NM_001314049.1 |
| GTTCATCAGCTGGGCACCTA | |||
| HIF-1α | TTCCTTCTCTTCTCCGCGTG/ | 175 | NM_1810542 |
| TGGCTGCATCTCGAGACTTTT | |||
| LIN28 | CCGAACCCCATGCGCACGTT/ | 137 | XM_011542148.2 |
| TTTGCAGGTGGCTGCGCCAAG | |||
| TGFβ | GTTCTTCAATGCGTCGGAGC/ | 214 | NM_001081849.1 |
| CACGACTCCGGTGACATCAA | |||
| p21 | AGAAGAGGCTGGTGGCTATTT/ | 169 | NM_001220777.1 |
| CCCGCCATTAGCGCATCAC | |||
| p53 | AGATAGCGATGGTCTGGC/ | 381 | NM_001126118.1 |
| TTGGGCAGTGCTCGCTTAGT | |||
| BAX | ACCAAGAAGCTGAGCGAGTGTC/ | 365 | XM_011527191.1 |
| ACAAAGATGGTCACGGTCTGCC | |||
| Cas-3 | GCGGTTGTAGAAGTTAATAAAGGT/ | 232 | NM_001354784.1 |
| CGACATCTGTACCAGACCGAG | |||
| TERT | CGGAAGAGTGTCTGGAGCAA | 107 | XM_011514106.1 |
| GGATGAAGCGGAGTCTGGA | |||
| MFN1 | GTTGCCGGGTGATAGTTGGA/ | 270 | XM_005247596.4 |
| TGCCACCTTCATGTGTCTCC | |||
| FIS1 | TGGTGCGGAGCAAGTACAAT/ | 251 | NM_016068.2 |
| TGCCCACGAGTCCATCTTTC | |||
| PRKN | GTGCAGAGACCGTGGAGAAA/ | 291 | XM_017010909.2 |
| GCTGCACTGTACCCTGAGTT | |||
| FOXO1 | ATTGAGCGCTTGGACTGTGA | 311 | XM_014732057.1 |
| CGCTGCCAAGTTTGACGAAA | |||
| GAPDH | GTCAGTGGTGGACCTGACCT/ | 256 | NM_001289746.1 |
| CACCACCCTGTTGCTGTAGC |
| Gene | Primer Sequence (5′→3′) | Accession Number |
|---|---|---|
| miR-24-3p | TGGCTCAGTTCAGCAGGAACAG | MIMAT0000080 |
| miR-17-5p | CAAAGTGCTTACAGTGCAGGTAG | MIMAT0000070 |
| miR-16-5p | TAGCAGCACGTAAATATTGGCG | MIMAT0000069 |
| miR-140-3p | TACCACAGGGTAGAACCACGGA | MIMAT0004597 |
| miR-146a-5p | TGAGAACTGAATTCCATGGGTT | MIMAT0000449 |
| miR-145-5p | GTCCAGTTTTCCCAGGAATCCCT | MIMAT0000437 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. https://doi.org/10.3390/jcm8060765
Alicka M, Major P, Wysocki M, Marycz K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. Journal of Clinical Medicine. 2019; 8(6):765. https://doi.org/10.3390/jcm8060765
Chicago/Turabian StyleAlicka, Michalina, Piotr Major, Michał Wysocki, and Krzysztof Marycz. 2019. "Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration" Journal of Clinical Medicine 8, no. 6: 765. https://doi.org/10.3390/jcm8060765
APA StyleAlicka, M., Major, P., Wysocki, M., & Marycz, K. (2019). Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. Journal of Clinical Medicine, 8(6), 765. https://doi.org/10.3390/jcm8060765