3. Results
Patients from the study group were aged 20 to 56 years, the average age was 29.9 ± 8.35 years, and half of the respondents were under 28 years of age. Subjects in the control group were aged 22 to 66 years, the average was 35.0 ± 10.9 years, and half of the respondents had already completed 34 years. Comparison of the mean age in the study and the control groups showed a statistically significant difference (p < 0.05). It turned out that the patients from the study group were significantly younger than in the control group.
In terms of TWI, masticatory muscle pain, and other bruxism symptoms in the oral cavity, all patients within the study group showed signs of tooth wear and muscle pain along with other symptoms. The majority of patients (30 out of 35 subjects) in the study group presented a score 1 of TWI, whereas the other five subjects of the study group presented a score 2 of TWI.
There was no statistically significant difference in the length of the examination between patients in the study group and control subjects (p > 0.05). In the study group, the average time between switching the device on and off was 7 h 2 min ± 1 h 8 min, and in the control group, it was 7 h 11 min ± 1 h 18 min.
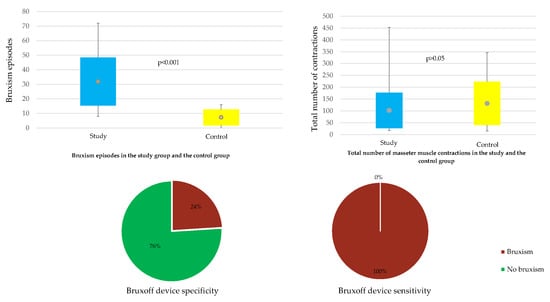
As expected, the comparison of the mean of the bruxism index in the study and the control group showed a statistically significant difference (
p < 0.001). It turned out that patients from the study group had a significantly larger mean bruxism index than those in the control group: 6.04 ± 2.55 vs. 1.35 ± 0.86 (
Table 3,
Figure 1).
Women dominated in each group. The respective percentages were: 65.7% and 80.0%. In the study group, the bruxism index in women yielded values from 2.60 to 12.5, the average was 5.90 ± 2.74, and in half of the respondents, it did not exceed 5.5 (
Table 4). In contrast, in men of the study group, the bruxism index ranged from 2.70 to 10.3, the mean was 6.30 ± 2.24, and in the half of respondents, it exceeded 5.95. The comparison of the bruxism index average values in the study group for men and women showed no statistically significant difference (
p > 0.05).
In the control group, the bruxism index in the women assumed values from 0.0 to 2.4 (the average was 1.27 ± 0.94), and in men of the control group, the bruxism index ranged from 1.2 to 2.1 (the average was 1.68 ± 0.37).
Comparison of the mean of the bruxism index in the control group for men and women did not show a statistically significant difference (p > 0.05).
There were no statistically significant correlations between the bruxism index and the sleep length of patients (p > 0.05) in both the study and control groups, and in this case, the Spearman’s rank correlation coefficient was even closer to zero.
Comparison of the mean heart rate in the study and control groups showed a statistically significant difference (
p < 0.05). It turned out that patients from the study group had an average heart rate lower than those in the control group: 59.6 ± 6.80 vs. 63.5 ± 4.86 beats per minute (
Table 5).
A comparison of the mean number of all contractions of masseter muscle in the study and in the control group did not show a statistically significant difference in this respect (
p > 0.05) (
Table 6).
Further, the comparison of the mean number of phase contractions in the study and in the control group did not show a statistically significant difference in this range (
p > 0.05) (
Table 7).
In this case, however, it is worth noting that the average number of phase contractions (RMMA) in the study and the control group was very similar, amounting to 23.5 ± 13.2 and 24.0 ± 22.1, respectively.
There was also no statistically significant difference between the analysed groups compared to the average number of tonic contractions in the traces of this study (
p > 0.05) (
Table 8).
The averages in the study and the control group turned out to be almost the same: 41.6 ± 52.5 and 41.7 ± 37.4. What draws attention is that there was a very large variation of this variable, especially in the study group. The coefficients of variation were, respectively, 126.0% and 89.8%. A comparison of the mean number of mixed contractions in the study and in the control group did not show a statistically significant difference (
p > 0.05) (
Table 9). The respective averages were: 4.06 ± 4.96 and 4.40 ± 6.14. It should be noted that there was a very large variation of this variable, both in the study and control groups. The coefficients of variation were, respectively, 122.2% and 139.5%. As can be seen, the volatility of the number of mixed contractions in both groups exceeded 100.0%.
There were no statistically significant correlations between the bruxism index and the total number of masseter muscle contractions in both the study group and the control group, and the correlation coefficients did not differ from zero in a statistically significant manner (p > 0.05).
There were also no statistically significant correlations between the bruxism index and the number of phase and mixed contractions, both in the study group and in the control group (
Table 10). Spearman’s rank correlation coefficients did not differ from zero in a statistically significant manner (
p > 0.05). However, a statistically significant relationship was found between the level of bruxism and the number of tonic contractions in the study group (
p < 0.05). It turned out that the higher the bruxism index, the more patients had tonic contractions. This relationship was moderately strong (Spearman’s rank correlation coefficient is 0.377,
p < 0.05). In the control group, however, no such relationship was found. In this case, the Spearman’s rank correlation coefficient was very close to zero.
In relation to the cut-off criteria for bruxism (
Table 11), data recorded with the Bruxoff device indicated that all subjects in the study group matched the criteria. Surprisingly, some subjects in the control group matched the criteria for bruxism, as well.
The sensitivity and specificity of Bruxoff were calculated (
Table 12). The sensitivity of the test is the ratio of true positive results to the sum of true positives and false negatives. The obtained sensitivity of 100% means that all subjects suffering from bruxism would be recognized using the Bruxoff device. The specificity of the test is the ratio of true negative results to the sum of true negative and false positives. The estimated specificity of the device would mean that 76% of all healthy people in the diagnostic test will be marked as healthy.
4. Discussion
Thorough masticatory muscle examination and observation of bruxism symptoms in the oral cavity underpin the bruxism diagnosis. Like mentioned before, every patient qualified for the study group had muscle pain and bruxism symptoms in the oral cavity upon examination, along with a positive self-report of bruxism. Subsequently, patients who qualified for the control group presented no muscle pain upon examination, no bruxism symptoms in the oral cavity, and a negative self-report for bruxism. As expected, the bruxism index (the amount of bruxism episodes per hour) was significantly higher for the study group. All patients with muscle pain and/or bruxism symptoms turned out to be bruxers. Interestingly, some patients without masticatory muscle pain and/or bruxism symptoms also turned out to be bruxers, as assessed by the Bruxoff device (
Table 12). This result could be subject to number of interpretations/explanations. It could be due to the positive and regulatory aspect of sleep bruxism in terms of providing an unobstructed airway for breathing in obstructive sleep apnea (OSA) and regulating salivation during sleep, as mentioned before. Therefore, patients showing no symptoms of sleep bruxism could still clench their teeth during some nights, but not to a degree that would cause them to show symptoms of sleep bruxism. Patients were not tested for OSA due to the limitations of the device. Nevertheless, this finding suggests that a bruxism diagnosis based solely on both EMG and ECG analysis could still overestimate SB [
58].
In terms of the accuracy of the Bruxoff device tested in this study, while the device turned out to have sensitivity of 100%, its specificity of 76% still leaves room for improvement, as there is a chance of providing a healthy subject with a false positive bruxism diagnosis. However, for this study to have a more thorough assessment of these parameters, the number of patients in both the study and the control group would have to be much larger. The producers of Bruxoff valued its sensitivity at 92% and its specificity at 85%. Other studies also showed the high sensitivity and specificity of the Bruxoff portable device (92.3% and 91.6%, respectively), as well as a high correlation and high agreement between Bruxoff and the PSG readings [
52,
58].
Moreover, the sensitivity and specificity outcome obtained in this study could suggest that the three maximum voluntary contractions (MVC) at the beginning of each examination were not performed by the patients with actual maximum strength. Therefore, this could mean that an examination performed while the patient is not watched over by a specialist could be limited, since there is no possibility of monitoring how the patient performs the examination with Bruxoff. According to The International Consensus of 2018 [
8], the cut-off points for establishing the presence or absence of bruxism should not be used in otherwise healthy individuals. Rather, bruxism-related masticatory muscle activities should be assessed in the behaviour’s continuum. Even though new research shows that bruxism could be connected with musculoskeletal pain, it does not support a direct and straight connection between them, but rather suggests a complex approach taking into account the presence of other risk factors [
59].
Research showed that bruxism affects women more commonly than men [
3,
60]. In this study, in terms of gender, no statistically significant difference between the study and the control groups was found. In each individual group, females dominated, and the male/female ratio in each group was not equal. However, this fact did not affect the mean bruxism index calculated separately for the men and women within each group. The limited number of participants was taken into account, while calculating all statistical differences, and, therefore, only highly visible statistical differences were considered significant (
p < 0.05). These findings are consistent with other papers that report no sex differences in terms of sleep bruxism [
1,
44,
61,
62,
63]. Moreover, the prevalence of women observed in studies on the subject results from their higher awareness and eagerness to seek professional help [
2].
In terms of the number and types of muscle contractions (tonic, phasic, and mixed) the results showed no significant differences between the study and the control group. A previous study showed the absence of a significant correlation between the number of masseter contraction per hour and the number of SB episodes per hour [
53]. In terms of a correlation between a specific type of contraction and the bruxism index, there was a surprisingly moderate correlation between the tonic (clenching) contractions and the bruxism index. The more tonic contractions there were, the higher the bruxism index. According to some literature, a higher amount of tonic activity could be associated with bruxism while awake and morning muscle symptoms [
45,
64,
65]. Therefore, it could be said that the morning muscle pain and fatigue reported by all patients within the study group were caused by clenching-type (tonic) sleep bruxism [
66]. However, other heavily supported research [
67,
68,
69,
70,
71] showed an increased number of phasic contractions in patients with sleep bruxism, which was not found in this study. This could suggest that the reliability of the Bruxoff device in this particular spectrum needs further study.
Surprisingly, the study group has showed a lower mean heart rate compared to the control group, even though episodes of sleep bruxism are said to be characterised by short tachycardic outbursts. This could be explained by the trigemino-cardiac response (TCR) that was triggered by episodes of sleep bruxism and which has been shown to cause bradycardia [
72,
73]. That, in turn, might have resulted in lower average heart rates in subjects from the study group. However, this finding could be also due to the fact that the ECG capabilities of Bruxoff are limited, since the detected heart rate signal was used only to identify the RMMA. As the producers of Bruxoff state, the device has not been designed and engineered for electrocardiographic investigations and is not suitable for diagnosing cardiovascular diseases. Moreover, such an outcome could have been produced by the limitations of the study, as the patients were not supervised when recording the data.
In terms of screening use, Bruxoff showed significant potential thanks to its EMG and ECG electrodes, which monitor both masseter muscle activity and the heart rate. A bruxism diagnosis based only on the surface EMG analysis tends to overestimate SB [
53]. These two parameters are, as previously mentioned in this paper, the foundations of the most valid theories pertaining to bruxism aetiology. Previous papers have also confirmed Bruxoff’s potential as a screening device for patients with sleep bruxism [
53,
54,
74]. In light of this, the authors suggested that the use of such portable EMG devices, e.g., Bruxoff (Bioelettronica, Italy), BiteStrip (Alldent, Australia), and GrindCare (Sunstar, Switzerland), could be considered by clinicians dealing with bruxism and could deliver promising results [
52,
53,
54,
75,
76,
77]. Further, devices measuring grinding, e.g., Bruxchecker (Scheu-Dental, Germany), should be taken into consideration [
78]. Perhaps these portable devices could someday challenge the “golden standard” that is PSG with audio and visual recording. However, even with the research results positioning portable EMG devices among the most reliable and easy to use methods for diagnosing bruxism, the complexity, sensitivity, and irreplaceable value of audio-video recordings in PSG still places it as the most detailed and valid diagnostic method for sleep bruxism. Furthermore, there have been developments in the form of portable sleep monitoring devices, e.g., the Nox-T3 Portable Sleep Monitor (Nox Medical, Reykjavik, Iceland), which seem to answer the need for simplified, yet detailed, diagnostic equipment in terms of sleep bruxism and sleep medicine in general [
6,
70].
The results of this study also showed a significant age difference between the groups, with the study group being much younger than the control group. Upon examining dozens of prospective participants, it became clear that, consistent with the epidemiologic data, sleep bruxism decreases with age—an occurrence that has been linked with coping mechanisms and responses to stress [
3,
79].
The Bruxoff study possesses several limitations that should be taken into account, like the large range of ages between the study and the control group. Further, due to the fact that Bruxoff does not have audio-video recording, it cannot register either sound or visual activity (e.g., grinding). Moreover, patients’ activities during sleep could possibly influence the Bruxoff readings. Due to the lack of EEG monitoring, the device cannot truly register patients’ sleep lengths, as there is no possibility of knowing, when the patient actually falls asleep and wakes up. The fact that the examination was performed for one night only might be an issue as the repeatability of the results could not be evaluated. Unfortunately, these two elements (audio–video and EEG) are crucial for a comprehensive and accurate diagnosis of sleep bruxism. Therefore, in spite of having EMG and ECG electrodes, Bruxoff cannot be considered a reliable match for polysomnography.
Bruxoff could be a useful device for dentists, who do not specialise in the field of temporomandibular disorders and whose experience with the clinical signs and symptoms of bruxism is limited. Furthermore, Bruxoff could be useful for doctors who conduct prosthodontic and periodontal treatments and are in need of an accurate method for screening and/or diagnosing their patients for bruxism.