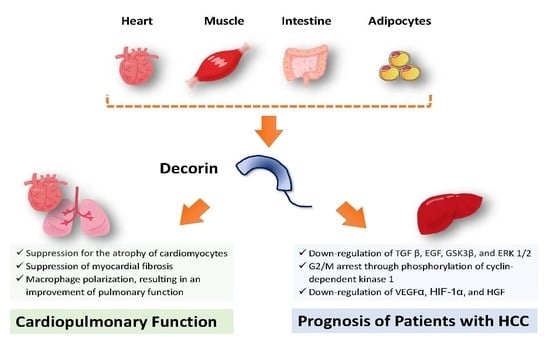
Impact of Decorin on the Physical Function and Prognosis of Patients with Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Subjects
2.4. Diagnosis, Barcelona Clinic Liver Cancer (BCLC) Staging, and Treatment of HCC
2.5. Measurement of Skeletal Muscle Index (SMI) and Visceral Fat Area
2.6. Measurement of Physical Function
2.7. Diagnosis of Sarcopenia
2.8. Biochemical Tests
2.9. Measurement of Serum Levels of Myostatin, FGF-21 and Decorin
2.10. Follow-Up and Definition of Survival Term
2.11. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Multivariate Correlation Analysis Between Serum Decorin Levels and Each Variable
3.3. Independent Factors Associated with Survival
3.4. Kaplan–Meier Analysis for Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weinmann, A.; Koch, S.; Niederle, I.M.; Schulze-Bergkamen, H.; Konig, J.; Hoppe-Lotichius, M.; Hansen, T.; Pitton, M.B.; Duber, C.; Otto, G.; et al. Trends in epidemiology, treatment, and survival of hepatocellular carcinoma patients between 1998 and 2009: An analysis of 1066 cases of a German HCC Registry. J. Clin. Gastroenterol. 2014, 48, 279–289. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Xu, J. Trends in Liver Cancer Mortality among Adults Aged 25 and Over in the United States, 2000–2016. NCHS Data Brief. 2018, 314, 1–8. [Google Scholar]
- Ikeda, K. Recent advances in medical management of hepatocellular carcinoma. Hepatol. Res. 2019, 49, 14–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Otsuka, Y.; Kawasaki, H.; Izumoto, H.; Ueki, H.; Kitahata, S.; Aibiki, T.; Okudaira, T.; Yamago, H.; Miyamoto, Y.; et al. Impact of muscle volume and muscle function decline in patients undergoing surgical resection for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2018, 33, 1271–1276. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Takahashi, A.; Hayashi, M.; Okai, K.; Abe, K.; Ohira, H. Skeletal muscle volume loss during transarterial chemoembolization predicts poor prognosis in patients with hepatocellular carcinoma. Hepatol. Res. 2019, 49, 778–786. [Google Scholar] [CrossRef]
- Takada, H.; Kurosaki, M.; Nakanishi, H.; Takahashi, Y.; Itakura, J.; Tsuchiya, K.; Yasui, Y.; Tamaki, N.; Takaura, K.; Komiyama, Y.; et al. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS ONE 2018, 13, e0198812. [Google Scholar] [CrossRef]
- Sawada, K.; Saitho, Y.; Hayashi, H.; Hasebe, T.; Nakajima, S.; Ikuta, K.; Fujiya, M.; Okumura, T. Skeletal muscle mass is associated with toxicity, treatment tolerability, and additional or subsequent therapies in patients with hepatocellular carcinoma receiving sorafenib treatment. JGH Open. 2019, 3, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, S.E.; Schlesinger, S.; Aleksandrova, K.; Jochem, C.; Jenab, M.; Gunter, M.J.; Overvad, K.; Tjonneland, A.; Boutron-Ruault, M.C.; Carbonnel, F.; et al. Association between physical activity and risk of hepatobiliary cancers: A multinational cohort study. J. Hepatol. 2019, 70, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Koya, S.; Hirota, K.; Goshima, N.; Yoshiyama, T.; Otsuka, T.; Bekki, M.; Iwanaga, S.; Nakano, D.; et al. Impact of Cancer Rehabilitation on the Prognosis of Patients with Hepatocellular Carcinoma. Oncol. Lett. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Dodig, M.; Muc, S.M.; Kalhan, S.C.; McCullough, A.J. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am. J. Physiol. Gastrointest Liver Physiol. 2004, 287, G1124–G1130. [Google Scholar] [CrossRef] [Green Version]
- Kanzleiter, T.; Rath, M.; Gorgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schurmann, A.; et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Bekki, M.; Hashida, R.; Kawaguchi, T.; Goshima, N.; Yoshiyama, T.; Otsuka, T.; Koya, S.; Hirota, K.; Matsuse, H.; Niizeki, T.; et al. The association between sarcopenia and decorin, an exercise-induced myokine, in patients with liver cirrhosis: A pilot study. J. Cachexia Sarcopenia Muscle Rapid Commun. 2018, 1, e00068. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Tateishi, R.; Koike, K. Proteoglycans Are Attractive Biomarkers and Therapeutic Targets in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018, 19, E3070. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Liang, W.; Yang, W.; Xia, R.; Song, Y. Decorin is responsible for progression of non-small-cell lung cancer by promoting cell proliferation and metastasis. Tumour Biol. 2015, 36, 3345–3354. [Google Scholar] [CrossRef]
- Dawoody Nejad, L.; Biglari, A.; Annese, T.; Ribatti, D. Recombinant fibromodulin and decorin effects on NF-kappaB and TGFbeta1 in the 4T1 breast cancer cell line. Oncol. Lett. 2017, 13, 4475–4480. [Google Scholar] [CrossRef]
- Horvath, Z.; Reszegi, A.; Szilak, L.; Danko, T.; Kovalszky, I.; Baghy, K. Tumor-specific inhibitory action of decorin on different hepatoma cell lines. Cell Signal. 2019, 62, 109354. [Google Scholar] [CrossRef]
- Svard, J.; Rost, T.H.; Sommervoll, C.E.N.; Haugen, C.; Gudbrandsen, O.A.; Mellgren, A.E.; Rodahl, E.; Ferno, J.; Dankel, S.N.; Sagen, J.V.; et al. Absence of the proteoglycan decorin reduces glucose tolerance in overfed male mice. Sci. Rep. 2019, 9, 4614. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xi, H.; Wei, B.; Cai, A.; Wang, T.; Wang, Y.; Zhao, X.; Song, Y.; Chen, L. Expression of decorin in intestinal tissues of mice with inflammatory bowel disease and its correlation with autophagy. Exp. Ther. Med. 2016, 12, 3885–3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubbiotti, M.A.; Neill, T.; Frey, H.; Schaefer, L.; Iozzo, R.V. Decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol. 2015, 48, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pohle, T.; Altenburger, M.; Shahin, M.; Konturek, J.W.; Kresse, H.; Domschke, W. Expression of decorin and biglycan in rat gastric tissue: Effects of ulceration and basic fibroblast growth factor. Scand. J. Gastroenterol. 2001, 36, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Arii, S.; Sata, M.; Sakamoto, M.; Shimada, M.; Kumada, T.; Shiina, S.; Yamashita, T.; Kokudo, N.; Tanaka, M.; Takayama, T.; et al. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol. Res. 2010, 40, 667–685. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address, e.e.e., European Association for the Study of the, L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Goto, E.; Matsuse, H.; Saito, H.; Hirota, K.; Taira, R.; Matsushita, Y.; Imanaga, M.; et al. Effects of in-hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol. Res. 2017, 47, E22–E34. [Google Scholar] [CrossRef] [Green Version]
- Hirota, K.; Kawaguchi, T.; Koya, S.; Nagamatsu, A.; Tomita, M.; Hashida, R.; Nakano, D.; Niizeki, T.; Matsuse, H.; Shiba, N.; et al. Clinical utility of the Liver Frailty Index for predicting muscle atrophy in chronic liver disease patients with hepatocellular carcinoma. Hepatol. Res. 2019, 50, 330–341. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS statement on six-minute walk test. Am. J. Respir Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef]
- Chong, C.D.; Dumkrieger, G.M.; Schwedt, T.J. Structural Co-Variance Patterns in Migraine: A Cross-Sectional Study Exploring the Role of the Hippocampus. Headache 2017, 57, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Gilabert, M.; Bruix, J.; Raoul, J.L. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2014, 11, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Tanaka, M.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Aino, H.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: Comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol. Res. 2019, 49, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachexia Sarcopenia Muscle. 2017, 8, 915–925. [Google Scholar] [CrossRef]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- Singhal, G.; Kumar, G.; Chan, S.; Fisher, F.M.; Ma, Y.; Vardeh, H.G.; Nasser, I.A.; Flier, J.S.; Maratos-Flier, E. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long term obesogenic diet. Mol. Metab. 2018, 13, 56–66. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Y.; Wang, W.; Yuan, Q.; Liu, Z.; Rasoul, L.M.; Wu, Q.; Liu, M.; Ye, X.; Li, D.; et al. Long-Term Administration of Fibroblast Growth Factor 21 Prevents Chemically-Induced Hepatocarcinogenesis in Mice. Dig. Dis. Sci. 2015, 60, 3032–3043. [Google Scholar] [CrossRef]
- Watanabe, M.; Singhal, G.; Fisher, F.M.; Beck, T.C.; Morgan, D.A.; Socciarelli, F.; Mather, M.L.; Risi, R.; Bourke, J.; Rahmouni, K.; et al. Liver-derived FGF21 is essential for full adaptation to ketogenic diet but does not regulate glucose homeostasis. Endocrine 2020, 67, 95–108. [Google Scholar] [CrossRef]
- Lai, J.; Chen, F.; Chen, J.; Ruan, G.; He, M.; Chen, C.; Tang, J.; Wang, D.W. Overexpression of decorin promoted angiogenesis in diabetic cardiomyopathy via IGF1R-AKT-VEGF signaling. Sci. Rep. 2017, 7, 44473. [Google Scholar] [CrossRef] [Green Version]
- Barallobre-Barreiro, J.; Gupta, S.K.; Zoccarato, A.; Kitazume-Taneike, R.; Fava, M.; Yin, X.; Werner, T.; Hirt, M.N.; Zampetaki, A.; Viviano, A.; et al. Glycoproteomics Reveals Decorin Peptides With Anti-Myostatin Activity in Human Atrial Fibrillation. Circulation 2016, 134, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.H.; Kim, M.; Bae, Y.K.; Kim, G.H.; Choi, S.J.; Oh, W.; Um, S.; Jin, H.J. Decorin Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Induces Macrophage Polarization via CD44 to Repair Hyperoxic Lung Injury. Int. J. Mol. Sci. 2019, 20, E4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, Z.; Kovalszky, I.; Fullar, A.; Kiss, K.; Schaff, Z.; Iozzo, R.V.; Baghy, K. Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol. 2014, 35, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y.; Du, Z.; Wang, Q.; Wu, M.; Wang, X.; Wang, L.; Cao, L.; Hamid, A.S.; Zhang, G. Recombinant human decorin suppresses liver HepG2 carcinoma cells by p21 upregulation. Onco Targets Ther. 2012, 5, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, A.S.; Li, J.; Wang, Y.; Wu, X.; Ali, H.A.; Du, Z.; Bo, L.; Zhang, Y.; Zhang, G. Recombinant human decorin upregulates p57KIP (2) expression in HepG2 hepatoma cell lines. Mol. Med. Rep. 2013, 8, 511–516. [Google Scholar] [CrossRef]
- Appunni, S.; Anand, V.; Khandelwal, M.; Gupta, N.; Rubens, M.; Sharma, A. Small Leucine Rich Proteoglycans (decorin, biglycan and lumican) in cancer. Clin. Chim. Acta. 2019, 491, 1–7. [Google Scholar] [CrossRef]
- Tsidulko, A.Y.; Kazanskaya, G.M.; Volkov, A.M.; Suhovskih, A.V.; Kiselev, R.S.; Kobozev, V.V.; Gaytan, A.S.; Krivoshapkin, A.L.; Aidagulova, S.V.; Grigorieva, E.V. Chondroitin sulfate content and decorin expression in glioblastoma are associated with proliferative activity of glioma cells and disease prognosis. Cell Tissue Res. 2020, 379, 147–155. [Google Scholar] [CrossRef]



| All Subjects | High Decorin | Low Decorin | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Range (min–max) | Median (IQR) | Range (min–max) | Median (IQR) | Range (min–max) | p | |
| Number (n) | 65 | N/A | 33 | N/A | 32 | N/A | N/A |
| Age (years) | 75 (71–80) | 60–90 | 76 (72–80) | 60–89 | 75 (71–80) | 63–90 | 0.9528 |
| Sex (women/men) | 38.5%/61.5% (25/40) | N/A | 54.5%/45.5% (18/15) | N/A | 21.9%/78.1% (7/25) | N/A | 0.0068 |
| Body mass index (kg/m2) | 23.9 (21.2–26.0) | 16.7–37.8 | 23.0 (21.5–26.3) | 19.6–30.3 | 24.1 (20.5–25.8) | 16.7–37.8 | 0.6227 |
| Hospitalization period (days) | 14 (11–21) | 7–55 | 13 (11.5–17.5) | 7–55 | 17 (11–21) | 7–34 | 0.3076 |
| Grip strength (kg) | 24.2 (20.3–31.3) | 13.3–42.8 | 24.1 (19.25–30.63) | 13.3–42.8 | 25.0 (21.5–32.1) | 14.9–39 | 0.8045 |
| Skeletal muscle index (cm2/m2) | 29.69 (23.94–35.20) | 11.85–51.18 | 28.50 (23.21–34.22) | 11.85–41.79 | 31.53 (24.33–36.82) | 12.45–51.18 | 0.3758 |
| Sarcopenia (Presence/Absence) | 18.5%/81.5% (12/53) | N/A | 6.1%/93.9% (2/31) | N/A | 31.3%/68.7% (10/22) | N/A | 0.0089 |
| Visceral fat area (cm2) | 61.7 (39.8–84.6) | 4.4–240.8 | 59.2 (39.8–78.1) | 24.2–240.8 | 61.9 (36.5–95.9) | 4.4–197.8 | 0.7578 |
| Serum creatine kinase (U/L) | 95 (70.5–132.5) | 17–374 | 99 (77–133) | 44–246 | 90.5 (63.75–129.5) | 17–374 | 0.3558 |
| 6-minute walking distance (m) | 379 (302–420) | 26–621 | 391 (365–433) | 228–621 | 334 (255–407) | 26–501 | 0.0093 |
| Decorin (pg/mL) | 17,322 (13,499–21,866) | 7400–32,102 | 21,799 (18,990–27,033) | 17,322–32,102 | 13,499 (11,939–14,726) | 7400–16,838 | <0.0001 |
| Myostatin (pg/mL) | 1699 (1180–3658) | 245–7788 | 3056 (1313–4610) | 501–6350 | 1500 (1154–3214) | 246–7788 | 0.0426 |
| FGF-21 (pg/mL) | 160 (116–344) | 13–2150 | 174 (140–340) | 39–2150 | 156 (96–357) | 13–1328 | 0.4197 |
| BCLC stage (A/B) | 10.8%/89.2% (7/58) | N/A | 15.2%/84.8%(5/28) | N/A | 6.2%/93.8%(2/30) | N/A | 0.2471 |
| AFP (ng/mL) | 32.75 (6.83–275.18) | 1.4–67,036 | 20 (8.25–72.27) | 3.9–1594 | 93 (5.5–1613) | 1.4–67,036 | 0.2318 |
| DCP (mAU/mL) | 76 (28–888.5) | 9–30,844 | 36 (24–211.5) | 12–17,353 | 144 (43–6753) | 9–30,844 | 0.0253 |
| Child–Pugh class (A/B) | 69.2%/30.8% (45/20) | N/A | 75.8%/24.2% (25/8) | N/A | 62.5%/37.5% (20/12) | N/A | 0.2469 |
| BCAA supplementation (With/Without) | 52.3%/47.7% (34/31) | N/A | 51.5%/48.5% (17/16) | N/A | 53.1%/46.9% (17/15) | N/A | 0.8966 |
| AST (IU/L) | 43 (32–55.5) | 19–158 | 45 (40–65.5) | 23–158 | 34 (26–48) | 19–99 | 0.0009 |
| ALT (IU/L) | 28 (21–37.5) | 7–186 | 32 (24.5–41) | 20–186 | 23 (17.5–32.5) | 7–87 | 0.0031 |
| ALP (IU/L) | 351 (291–479) | 180–854 | 356 (309.5–541.5) | 200–854 | 325 (275.75–455) | 180–659 | 0.2299 |
| GGT (IU/L) | 44 (26–73.5) | 9–551 | 45 (26.5–79.5) | 15–551 | 42 (26–66.5) | 9–252 | 0.6088 |
| Total protein (g/dL) | 7.28 (6.72–7.78) | 5.94–8.89 | 7.36 (6.62–7.81) | 5.94–8.15 | 7.28 (6.82–7.62) | 6.04–8.89 | 0.9477 |
| Albumin (g/dL) | 3.5 (3.1–3.7) | 2.5–4.3 | 3.4 (3.1–3.7) | 2.5–4.3 | 3.5 (3.0–3.8) | 2.8–4.2 | 1.0000 |
| Total bilirubin (mg/dL) | 0.9 (0.6–1.3) | 0.3–2.8 | 0.9 (0.6–1.3) | 0.4–2.8 | 0.9 (0.6–1.2) | 0.3–1.6 | 0.5996 |
| Prothrombin activity (%) | 80 (68–88) | 38–117 | 81 (65.5–90) | 42–117 | 79 (69–85.5) | 38–108 | 0.6365 |
| Blood urea nitrogen (mg/dL) | 17 (14–19.6) | 5.9–47.6 | 14.9 (13–18.9) | 5.9–28.1 | 17.45 (15–20.18) | 11.5–47.6 | 0.0253 |
| Creatinine (mg/dL) | 0.74 (0.61–0.92) | 0.43–1.91 | 0.66 (0.55–0.77) | 0.43–1.52 | 0.81 (0.66–1.03) | 0.56–1.91 | 0.0013 |
| eGFR (mL/min/1.73 m2) | 73.2 (54.3–84.85) | 27.3–121.3 | 78.6 (62.15–89.95) | 34.3–121.3 | 65.7 (52.63–75.8) | 27.3–102.4 | 0.0107 |
| Total cholesterol (mg/dL) | 144 (126–162) | 79–233 | 138 (121–156) | 84–197 | 147 (128–163) | 79–233 | 0.3154 |
| Triglyceride (mg/dL) | 82 (70–108) | 28–249 | 75 (64–94) | 28–249 | 91 (78–130) | 54–179 | 0.0237 |
| HbA1c (%) | 5.8 (5.5–6.4) | 4.3–13.4 | 5.7 (5.25–6.1) | 4.3–8.3 | 6.1 (5.7–6.8) | 4.7–13.4 | 0.0268 |
| Red blood cell count (×104/µL) | 389 (355–420) | 249–615 | 385 (356–416) | 310–455 | 393 (347–442) | 249–615 | 0.7528 |
| Hemoglobin (g/dL) | 11.9 (10.4–12.75) | 7.3–15.4 | 11.9 (10.6–12.8) | 7.3–15.4 | 11.9 (9.8–12.7) | 7.3–14.9 | 0.7083 |
| White blood cell count (/µL) | 3800 (3100–5050) | 1800–7900 | 3700 (3050–5560) | 1900–6600 | 4150 (3125–5525) | 1800–7900 | 0.2270 |
| Platelet count (×103/mm3) | 10.9 (8.35–15.1) | 3.2–31.8 | 9.4 (7.8–13.0) | 3.2–22.6 | 11.8 (8.6–16.1) | 4.0–31.8 | 0.0881 |
| Variable | Correlation Coefficient | p |
|---|---|---|
| Age | −0.0250 | 0.8750 |
| Body mass index | 0.0415 | 0.7942 |
| Grip strength | −0.0532 | 0.7380 |
| Skeletal muscle index | −0.1362 | 0.3898 |
| Visceral fat area | 0.0278 | 0.861 |
| 6-min walking distance | 0.2927 | 0.0353 |
| Creatine kinase | −0.0062 | 0.9690 |
| Myostatin | 0.3200 | 0.0389 |
| FGF-21 | −0.0352 | 0.8249 |
| AFP | −0.2270 | 0.1482 |
| DCP | −0.3476 | 0.0241 |
| AST | 0.2453 | 0.0992 |
| ALT | 0.2734 | 0.0798 |
| ALP | 0.1260 | 0.4266 |
| GGT | 0.0042 | 0.979 |
| Total protein | −0.0197 | 0.9015 |
| Albumin | −0.1754 | 0.2664 |
| Total bilirubin | 0.1054 | 0.5063 |
| Prothrombin activity | 0.1078 | 0.4968 |
| Blood urea nitrogen | −0.1606 | 0.3095 |
| Creatinine | −0.1650 | 0.2965 |
| eGFR | 0.0695 | 0.6617 |
| Total cholesterol | −0.0914 | 0.5650 |
| Triglyceride | −0.0594 | 0.7089 |
| HbA1c | −0.2748 | 0.0782 |
| Red blood cell count | −0.1337 | 0.3984 |
| Hemoglobin | −0.0384 | 0.8091 |
| White blood cell count | −0.233 | 0.1376 |
| Platelet count | −0.2261 | 0.15 |
| Factors | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Decorin (High/Low) | 2.808 | 1.016–8.018 | 0.0498 |
| BCLC stage (A/B–C) | 6.720 | 0.707–73.877 | 0.0553 |
| Child–Pugh class (A/B) | 1.436 | 0.461–4.473 | 0.5308 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, T.; Yoshio, S.; Sakamoto, Y.; Hashida, R.; Koya, S.; Hirota, K.; Nakano, D.; Yamamura, S.; Niizeki, T.; Matsuse, H.; et al. Impact of Decorin on the Physical Function and Prognosis of Patients with Hepatocellular Carcinoma. J. Clin. Med. 2020, 9, 936. https://doi.org/10.3390/jcm9040936
Kawaguchi T, Yoshio S, Sakamoto Y, Hashida R, Koya S, Hirota K, Nakano D, Yamamura S, Niizeki T, Matsuse H, et al. Impact of Decorin on the Physical Function and Prognosis of Patients with Hepatocellular Carcinoma. Journal of Clinical Medicine. 2020; 9(4):936. https://doi.org/10.3390/jcm9040936
Chicago/Turabian StyleKawaguchi, Takumi, Sachiyo Yoshio, Yuzuru Sakamoto, Ryuki Hashida, Shunji Koya, Keisuke Hirota, Dan Nakano, Sakura Yamamura, Takashi Niizeki, Hiroo Matsuse, and et al. 2020. "Impact of Decorin on the Physical Function and Prognosis of Patients with Hepatocellular Carcinoma" Journal of Clinical Medicine 9, no. 4: 936. https://doi.org/10.3390/jcm9040936
APA StyleKawaguchi, T., Yoshio, S., Sakamoto, Y., Hashida, R., Koya, S., Hirota, K., Nakano, D., Yamamura, S., Niizeki, T., Matsuse, H., & Torimura, T. (2020). Impact of Decorin on the Physical Function and Prognosis of Patients with Hepatocellular Carcinoma. Journal of Clinical Medicine, 9(4), 936. https://doi.org/10.3390/jcm9040936