Reliability, Factor Structure and Predictive Validity of the Widespread Pain Index and Symptom Severity Scales of the 2010 American College of Rheumatology Criteria of Fibromyalgia
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Assessment Instruments
2.3. Pain Induction and Quantification
2.4. Procedure
2.5. Statistical Analysis
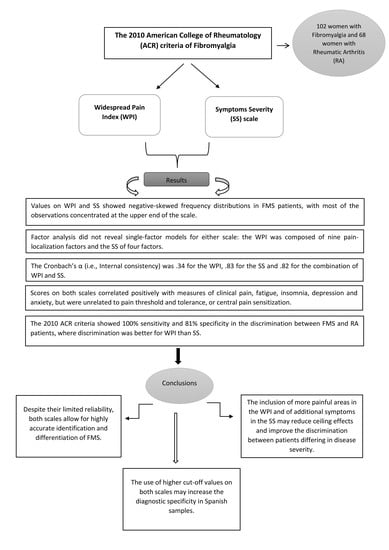
3. Results
3.1. Demographic and Clinical Data of FMS and RA Patients
3.2. Frequency Distribution of WPI and SS Scores
3.3. Factor Structure of the WPI and SS
3.4. Internal Consistency of the WPI and SS Scales
3.5. Associations of the WPI and SS Scores with Clinical Symptoms
3.6. Associations between WPI, SS and Evoked Pain Measures
3.7. Ability of WPI and SS to Differentiate between FMS and RA Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.; Abeles, M.; Clark, P.; et al. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Nijs, J.; Meeus, M.; Van Oosterwijck, J.; Roussel, N.; De Kooning, M.; Ickmans, K.; Matic, M. Treatment of central sensitization in patients with ‘unexplained’ chronic pain: What options do we have? Expert Opin. Pharmacother. 2011, 12, 1087–1098. [Google Scholar] [CrossRef]
- Staud, R.; Koo, E.; Robinson, M.E.; Price, D.D. Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain 2007, 130, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Davis, F.; Gostine, M.; Roberts, B.; Risko, R.; Cappelleri, J.C.; Sadosky, A. Characterizing classes of fibromyalgia within the continuum of central sensitization syndrome. J. Pain Res. 2018, 11, 2551–2560. [Google Scholar] [CrossRef] [Green Version]
- De La Coba, P.; Bruehl, S.; Padilla, M.M.; Del Paso, G.A.R. Responses to slowly repeated evoked pain stimuli in fibromyalgia patients: Evidence of enhanced pain sensitization. Pain Med. 2017, 18, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- de la Coba, P.; Bruehl, S.; Galvez-Sánchez, C.M. Specificity of slowly repeated evoked pain in comparison with traditional pain threshold and tolerance measures in fibromyalgia patients. Psychosom. Med. 2018, 80, 573–580. [Google Scholar]
- Gracely, R.H.; Petzke, F.; Wolf, J.M.; Clauw, D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002, 46, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Montoro, C.I.; Del Paso, G.A.R.; Duschek, S. Alexithymia in fibromyalgia syndrome. Pers. Individ. Differ. 2016, 102, 170–179. [Google Scholar] [CrossRef]
- Del Paso, G.A.R.; Garrido, S.; Pulgar, Á.; Duschek, S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J. Psychosom. Res. 2011, 70, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Moyano, S.; Kilstein, J.G.; de Miguel, C.A. Nuevos criterios diagnósticos de fibromialgia: Vinieron para quedarse? Reumatol. Clin. 2014, 3, 716–721. [Google Scholar] [CrossRef]
- Okifuji, A.; Hare, B.D. Management of fibromyalgia syndrome: Review of evidence. Pain Ther. 2013, 2, 87–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, R.M. Fibrositis: Misnomer for a common rheumatic disorder. West. J. Med. 1981, 134, 405–413. [Google Scholar] [PubMed]
- Hudson, J.I.; Hudson, M.S.; Pliner, L.F.; Goldenberg, D.L.; Pope, H.G. Fibromyalgia and major affective disorder: A controlled phenomenology and family history study. Am. J. Psychiatry 1985, 142, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gracely, R.H.; Geisser, M.E.; Giesecke, T.; Grant, M.A.B.; Petzke, F.; Williams, D.A.; Clauw, D.J. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004, 127, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turk, D.C.; Flor, H. Primary fibromyalgia is greater than tender points: Toward a multiaxial taxonomy. J. Rheumatol. Suppl. 1989, 19, 80–86. [Google Scholar] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Rheum. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, F.; Clauw, D.; Fitzcharles, M.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Usui, C.; Hatta, K.; Aratani, S.; Yagishita, N.; Nishioka, K.; Kanazawa, T.; Ito, K.; Yamano, Y.; Nakamura, H.; Nakajima, T.; et al. The Japanese version of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and the fibromyalgia symptom scale: Reliability and validity. Mod. Rheumatol. 2012, 22, 40–44. [Google Scholar] [CrossRef]
- Fitzcharles, M.; Ste-Marie, P.A.; Panopalis, P.; A Ménard, H.; Shir, Y.; Wolfe, F. The 2010 American college of rheumatology fibromyalgia survey diagnostic criteria and symptom severity scale is a valid and reliable tool in a French speaking fibromyalgia cohort. BMC Musculoskelet. Disord. 2012, 13, 179. [Google Scholar] [CrossRef] [Green Version]
- Yanmaz, M.N.; Atar, S.; Biçer, M. The reliability and validity of the Turkish version of fibromyalgia survey diagnostic criteria and symptom severity scale. J. Back Musculoskelet. Rehabil. 2016, 29, 287–293. [Google Scholar] [CrossRef]
- Ghavidel-Parsa, B.; Bidari, A.; A Maafi, A.; Hassankhani, A.; Hajiabbasi, A.; Montazeri, A.; Sanaei, O.; Ghalehbaghi, B. The impact of fibromyalgia on health status according to the types, demographic background and pain index. Clin. Exp. Rheumatol. 2016, 34, 134–139. [Google Scholar]
- Segura-Jiménez, V.; Aparicio, V.A.; Gallardo, I.C.; Álvarez; Soriano-Maldonado, A.; Estévez-López, F.; Delgado-Fernández, M.; Carbonell-Baeza, A. Validation of the modified 2010 American College of Rheumatology diagnostic criteria for fibromyalgia in a Spanish population. Rheumatology 2014, 53, 1803–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S. Healey, criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- First, M.; Spitzer, R.L.; Gibbon, M. Entrevista Clínica Estructurada para los Trastornos del Eje I del DSM-IV: SCIDI; Versión Clínica; Madsson: Barcelona, Spain, 1999. [Google Scholar]
- Casanueva, B.; García-Fructuoso, F.; Belenguer, R.; Alegre, C.; Moreno-Muelas, J.; Hernández, J.; Pina, T.; González-Gay, M. The Spanish version of the 2010 American College of Rheumatology preliminary clinical diagnostic criteria for fibromyalgia: Reliability and validity assessment. Exp. Rheumatol. 2016, 34, S55–S58. [Google Scholar]
- Lázaro, C.; Bosch, F.; Torrubia, R.; Josep-Eladi, B. The development of a Spanish Questionnaire for assessing pain: Preliminary data concerning reliability and validity. Eur. J. Psychol. Assess. 1994, 10, 145–151. [Google Scholar]
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975, 1, 277–299. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.; Lushene, R.E. Manual del Cuestionario de Ansiedad Estado/Rasgo (STAI); TEA Ediciones: Madrid, España, 1982. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Palo Alto, Consulting Psychologist: Palo Alto, CA, USA, 1970. [Google Scholar]
- Vázquez, C.; Sanz, J. Fiabilidad y validez de la versión española del Inventario para la epresión de Beck de 1978 en pacientes con trastornos psicológicos. Clin. Salud. 1999, 1, 59–81. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Bulbena, A.; Berrios, G.E.; Fernández de Larrinoa, P. Medición Clínica en Psiquiatría y Psicología; Madrid; Masson: Madrid, Spain, 2000. [Google Scholar]
- Krupp, L.B.; La Rocca, N.G.; Muir-Nash, J. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Bobes, J.; González, M.P.; Sáiz, P.A. Propiedades psicométricas del cuestionario Oviedo de sueño. Psicothema 2000, 1, 107–112. [Google Scholar]
- Rodriguez, L.; Cano, E.J.; Blanco, A. Evaluación de las estrategias de afrontamiento de dolor crónico. Actas Esp. Psiquiatría. 2004, 32, 82–91. [Google Scholar]
- Rosenstiel, A.K.; Keefe, F.J. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain 1983, 17, 33–44. [Google Scholar] [CrossRef]
- Del Paso Reyes, G.A.; Montoro, C.; De Guevara Muñoz-Ladrón, C.; Duschek, S.; Jennings, J.R.; Aguilar, C.I.M. The effect of baroreceptor stimulation on pain perception depends on the elicitation of the reflex cardiovascular response: Evidence of the interplay between the two branches of the baroreceptor system. Boil. Psychol. 2014, 101, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Comrey, A.L. Manual de Análisis Factorial; Madrid; Cátedra: Madrid, Spain, 1985. [Google Scholar]
- Moyano, S.; Scolnik, M.; Vergara, F.; Garcia, M.V.; Sabelli, M.R.; Rosa, J.E.; Catoggio, L.J.; Soriano, E.R. Evaluation of learned helplessness, perceived self-efficacy, and functional capacity in patients with fibromyalgia and rheumatoid arthritis. J. Clin. Rheumatol. 2019, 25, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Boateng, G.O.; Neilands, T.B.; Frongillo, E.A.; Melgar-Quiñonez, H.R.; Young, S.L. Best practices for developing and validating scales for health, social, and behavioral research: A primer. Front. Public Heal. 2018, 6, 149. [Google Scholar] [CrossRef]
- Faro, M.; Sáez-Francàs, N.; Castro-Marrero, J. Impact of the fibromyalgia in the chronic fatigue syndrome. Med. Clin. 2014, 142, 519–525. [Google Scholar] [CrossRef]
- Kamping, S.; Bomba, I.C.; Kanske, P.; Diesch, E.; Flor, H. Deficient modulation of pain by a positive emotional context in fibromyalgia patients. Pain 2013, 154, 1846–1855. [Google Scholar] [CrossRef]
- Watson, D.; Pennebaker, J.W. Health complaints, stress and distress: Exploring the central role of negative affectivity. Psychol. Rev. 1989, 96, 234–254. [Google Scholar] [CrossRef]
- Campos, R.; Vázquez, I. The impact of Fibromyalgia on health-related quality of life in patients according to age. Rheumatol. Int. 2012, 33, 1419–1424. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, K.-E.; Park, D.-J.; Kim, S.-H.; Nah, S.-S.; Lee, J.H.; Kim, S.-K.; Lee, Y.-A.; Hong, S.-J.; Kim, H.-S.; et al. Determinants of quality of life in patients with fibromyalgia: A structural equation modeling approach. PLoS ONE 2017, 12, e0171186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoro, C.I.; Del Paso, G.A.R. Personality and fibromyalgia: Relationships with clinical, emotional, and functional variables. Pers. Individ. Differ. 2015, 85, 236–244. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Del Paso Reyes, G.A.; Duschek, S. Cognitive impairments in fibromyalgia syndrome: Associations with positive and negative affect, alexithymia, pain catastrophizing and self-esteem. Front. Psychol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisser, M.E.; Gracely, R.H.; Giesecke, T.; Petzke, F.W.; Williams, D.A.; Clauw, D.J. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur. J. Pain 2007, 11, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, J.L.B.; Fernández, T.V.S.; Rodríguez, L.A.; Muñiz, J.; Lemos-Giráldez, S.; Fernández, A.A. Sleep architecture in patients with fibromyalgia. Psicothema 2011, 23, 368–373. [Google Scholar]
- Bidari, A.; Hassanzadeh, M.; Parsa, B.G.; Kianmehr, N.; Kabir, A.; Pirhadi, S.; Sayfi, M.; Toutounchi, M.; Fattahi, F.; Karimi, F.Z. Validation of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia in an Iranian population. Rheumatol. Int. 2013, 33, 2999–3007. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Del Paso, G.A.R. Diagnostic criteria for fibromyalgia: Critical review and future perspectives. J. Clin. Med. 2020, 9, 1219. [Google Scholar] [CrossRef]
- Arnold, L.M.; Bennett, R.; Crofford, L.J.; Dean, L.E.; Clauw, D.; Goldenberg, D.L.; Fitzcharles, M.; Paiva, E.S.; Staud, R.; Sarzi-Puttini, P.; et al. AAPT Diagnostic criteria for fibromyalgia. J. Pain 2018, 20, 611–628. [Google Scholar] [CrossRef] [Green Version]
- Fillingim, R.B.; Bruehl, S.; Dworkin, R.H.; Dworkin, S.F.; Loeser, J.D.; Turk, D.C.; Widerstrom-Noga, E.; Arnold, L.; Bennett, R.; Edwards, R.R.; et al. The ACTTION-American Pain Society Pain Taxonomy (AAPT): An evidence-based and multidimensional approach to classifying chronic pain conditions. J. Pain 2014, 15, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, R.H.; Bruehl, S.; Fillingim, R.B.; Loeser, J.D.; Terman, G.W.; Turk, D.C. Multidimensional diagnostic criteria for chronic pain: Introduction to the ACTTION–American Pain Society Pain Taxonomy (AAPT). J. Pain 2016, 17, T1–T9. [Google Scholar] [CrossRef] [Green Version]
- Salaffi, F.; Di Carlo, M.; Farah, S. Diagnosis of fibromyalgia: Comparison of the 2011/2016 ACR and AAPT criteria and validation of the modified fibromyalgia assessment status. Rheumatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Brähler, E.; Ablin, J.; Wolfe, F. 2016 modified American College of Rheumatology fibromyalgia criteria, ACTTION-APS Pain Taxonomy criteria and the prevalence of fibromyalgia. Arthritis Rheum. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F. Letter to the editor, “Fibromyalgia Criteria”. J. Pain 2019, 20, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Duffield, S.J.; Miller, N.; Zhao, S.S.; Goodson, N.J. Concomitant fibromyalgia complicating chronic inflammatory arthritis: A systematic review and meta-analysis. Rheumatology 2018, 57, 1453–1460. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Bingham, C.O.; Edwards, R.R. Pain sensitization is associated with disease activity in rheumatoid arthritis patients: A cross-sectional study. Arthritis Care Res. 2018, 70, 197–204. [Google Scholar] [CrossRef]


| FMS | RA | U / χ2 | p | |
|---|---|---|---|---|
| Age | 52.44 ± 7.91 | 51.73 ± 10.98 | 3224.5 | 0.89 |
| Body mass index (BMI) | 28.20 ± 5.21 | 27.73 ± 5.32 | 3221.0 | 0.43 |
| Years of Education | 10.74 ± 4.27 | 10.25 ± 4.08 | 3400.5 | 0.82 |
| Depression (%) | 87 (85.29%) | 20 (29.41%) | 50.14 | p < 0.01 |
| Anxiety disorders* (%) | 93 (91.18%) | 27 (39.71%) | 47.11 | p < 0.01 |
| Antidepressant use (%) | 88 (86.27%) | 17 (25%) | 60.32 | p < 0.01 |
| Anxiolytic use (%) | 85 (83.33%) | 20 (29.41%) | 45.90 | p < 0.01 |
| Non-opioids analgesic use (%) | 85 (83.33%) | 51 (75%) | 0.35 | 0.67 |
| Opiate use (%) | 56 (54.90%) | 19 (27.94%) | 10.48 | 0.001 |
| Widespread Pain Index pain areas (WPI) | 17.84 ± 1.60 | 5.82 ± 4.86 | 223.5 | p < 0.01 |
| Symptom Severity fatigue (SS2a) | 2.52 ± 0.54 | 1.42 ± 1.17 | 1692.0 | p < 0.01 |
| Symptom Severity waking unrefreshed (SS2a) | 2.43 ± 0.64 | 1.06 ± 1.24 | 1502.0 | p < 0.01 |
| Symptom Severity cognitive symptoms (SS2a) | 2.41 ± 0.67 | 0.94 ± 1.06 | 1070.0 | p < 0.01 |
| Subtotal Symptom Severity (SS2a) | 7.39 ± 1.47 | 3.42 ± 2.91 | 964.5 | p < 0.01 |
| Total of symptoms (SS2b) | 27.27 ± 6.64 | 4.87 ± 4.08 | 27.50 | p < 0.01 |
| Range of Symptoms (SS2b) | 2.65 ± 0.48 | 1.09 ± 0.29 | 108.0 | p < 0.01 |
| Total Symptom Severity (SS) | 10.05 ± 1.73 | 4.51 ± 3.05 | 512.0 | p < 0.01 |
| Slowly Repeated Evoked Pain (SREP) | 1.41 ± 1.31 | 0.05 ± 0.74 | 842.5 | p < 0.01 |
| Threshold (kg) | 2.20 ± 1.39 | 3.40 ± 1.80 | 1872.5 | p < 0.01 |
| Tolerance (kg) | 6.08 ± 3.16 | 7.94 ± 2.63 | 1978.5 | p < 0.01 |
| Total Pain (MPQ) | 79.71 ± 35.23 | 44.60 ± 33.02 | 1441.5 | p < 0.01 |
| Current Pain Intensity (MPQ) | 3.66 ± 1.12 | 2.94 ± 1.07 | 2231.5 | p < 0.01 |
| Emotional Pain (MPQ) | 11.51 ± 7.21 | 6.96 ± 7.15 | 2052.0 | p < 0.01 |
| Sensorial Pain (MPQ) | 51.25 ± 23.77 | 27.27 ± 22.10 | 1385.5 | p < 0.01 |
| State-Anxiety (STAI-E) | 23.18 ± 10.42 | 16.12 ± 13.46 | 2524.5 | p < 0.01 |
| Trait-Anxiety (STAI-T) | 40.88 ± 13.45 | 28.99 ± 14.17 | 865.5 | p < 0.01 |
| Depression (BDI) | 42.69 ± 14.21 | 16.76 ± 15.29 | 807.0 | p < 0.01 |
| Fatigue (FSS) | 52.86 ± 9.27 | 38.01 ± 17.97 | 1755.0 | p < 0.01 |
| Insomnia (COS) | 36.56 ± 12.12 | 20.88 ± 14.97 | 1661.5 | p < 0.01 |
| Catastrophizing (CSQ) | 25.73 ± 11.16 | 11.97 ± 12.33 | 1382.0 | p < 0.01 |
| WPI | Factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Shoulder girdle, left | 0.85 | ||||||||
| Shoulder girdle, right | 0.87 | ||||||||
| Upper arm, left | 0.63 | ||||||||
| Lower arm, left | 0.60 | ||||||||
| Upper back | 0.81 | ||||||||
| Lower back | 0.81 | ||||||||
| Upper leg left | 0.75 | ||||||||
| Jaw right | 0.69 | ||||||||
| Lower arm, right | 0.66 | ||||||||
| Hip (buttock) left | 0.92 | ||||||||
| Hip (buttock) right | 0.65 | ||||||||
| Chest | 0.63 | ||||||||
| Abdomen | 0.76 | ||||||||
| Upper leg right | 0.84 | ||||||||
| Lower leg left | 0.55 | ||||||||
| Jaw left | 0.70 | ||||||||
| Neck | 0.91 | ||||||||
| Upper arm, right | 0.81 | ||||||||
| Lower leg right | 0.90 | ||||||||
| SS | Factors | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Dizziness | 0.57 | |||
| Insomnia | 0.65 | |||
| Depression | 0.69 | |||
| Constipation | 0.65 | |||
| Pain in upper abdomen | 0.74 | |||
| Nausea | 0.68 | |||
| Nervousness | 0.74 | |||
| Chest pain | 0.81 | |||
| Blurred vision | 0.50 | |||
| Fever | 0.61 | |||
| Diarrhea | 0.49 | |||
| Dry mouth | 0.59 | |||
| Wheezing | 0.38 | |||
| Muscle Pain | 0.53 | |||
| Irritable bowel syndrome | 0.38 | |||
| Fatigue/Tiredness | 0.67 | |||
| Thinking or remembering problem | 0.74 | |||
| Muscle Weakness | 0.81 | |||
| Headache | 0.67 | |||
| Pain/cramps in abdomen | 0.62 | |||
| Numbness/tingling | 0.57 | |||
| Hair loss | −0.32 | |||
| Raynauld’s | 0.45 | |||
| Ringing in ears | 0.51 | |||
| Heartburn | 0.33 | |||
| Loss/change in taste | 0.56 | |||
| Shortness of breath | 0.50 | |||
| Loss of appetite | 0.65 | |||
| Rash | 0.46 | |||
| Hearing difficulties | 0.46 | |||
| Easy bruising | 0.44 | |||
| Frequent urination | 0.36 | |||
| Bladder spasms | 0.33 | |||
| Hives/welts | 0.31 | |||
| Itching | 0.46 | |||
| Oral ulcers | 0.63 | |||
| Seizures | 0.52 | |||
| Dry eyes | 0.59 | |||
| Sun sensitivity | 0.56 | |||
| ACR Diagnostic Criteria | SREP | Threshold | Tolerance | TP | CPI | EP | SP | FSS | COS |
|---|---|---|---|---|---|---|---|---|---|
| WPI | −0.09 | −0.01 | 0.04 | 0.24 + | 0.37 * | 0.27 * | 0.22 + | 0.12 | 0.23 + |
| SS Fatigue (SS2a) | −0.16 | −0.03 | 0.05 | 0.09 | 0.34 * | 0.13 | 0.06 | 0.22 + | 0.17 |
| SS Waking Unrefreshed (SS2a) | −0.12 | −0.21 + | −0.10 | 0.31 * | 0.35 * | 0.33 * | 0.26 * | 0.12 | 0.11 |
| SS Cognitive Symptoms (SS2a) | −0.08 | −0.13 | −0.16 | 0.13 | 0.19 | 0.19 | 0.05 | 0.22 + | 0.19 |
| Subtotal SS (SS2a) | −0.16 | −0.14 | −0.10 | 0.26 * | 0.40 * | 0.32 * | 0.19 | 0.21 + | 0.14 |
| Total of symptoms (SS2b) | −0.26 + | −0.12 | 0.04 | 0.49 * | 0.34 * | 0.39 * | 0.48 * | 0.28 * | 0.18 |
| Range of Symptoms (SS2b) | −0.32 * | −0.05 | 0.06 | 0.50 * | 0.26 * | 0.41 * | 0.46 * | 0.25 + | 0.09 |
| Total Symptom Severity (SS) | −0.22 + | −0.11 | −0.07 | 0.34 * | 0.42 * | 0.35 * | 0.27 * | 0.24 + | 0.15 |
| ACR Diagnostic Criteria | State-Anxiety (STAI-E) | Trait-Anxiety (STAI-T) | Depression (BDI) | Catastrophizing (CSQ) |
|---|---|---|---|---|
| Widespread Pain Index pain areas (WPI) | −0.01 | 0.15 | 0.19 | −0.11 |
| Symptom Severity fatigue (SS2a) | −0.12 | 0.02 | 0.03 | 0.03 |
| Symptom Severity waking unrefreshed (SS2a) | −0.03 | 0.09 | 0.23 + | 0.22 + |
| Symptom Severity cognitive symptoms (SS2a) | −0.01 | 0.11 | 0.11 | 0.04 |
| Subtotal Symptom Severity (SS2a) | −0.07 | 0.11 | 0.21 + | 0.11 |
| Total of symptoms (SS2b) | 0.01 | 0.26 * | 0.34 * | 0.20 + |
| Range of Symptoms (SS2b) | −0.10 | 0.30 * | 0.30 * | 0.23 + |
| Total Symptom Severity (SS) | −0.08 | 0.16 | 0.22 + | 0.14 |
| WPI | SS | WPI + SS | |
|---|---|---|---|
| Sensitivity | 100 | 89.2 | 100 |
| Specificity | 89.7 | 83.8 | 100 |
| Overall Accuracy | 95.9 | 87.1 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Sánchez, C.M.; de la Coba, P.; Duschek, S.; Reyes del Paso, G.A. Reliability, Factor Structure and Predictive Validity of the Widespread Pain Index and Symptom Severity Scales of the 2010 American College of Rheumatology Criteria of Fibromyalgia. J. Clin. Med. 2020, 9, 2460. https://doi.org/10.3390/jcm9082460
Galvez-Sánchez CM, de la Coba P, Duschek S, Reyes del Paso GA. Reliability, Factor Structure and Predictive Validity of the Widespread Pain Index and Symptom Severity Scales of the 2010 American College of Rheumatology Criteria of Fibromyalgia. Journal of Clinical Medicine. 2020; 9(8):2460. https://doi.org/10.3390/jcm9082460
Chicago/Turabian StyleGalvez-Sánchez, Carmen M., Pablo de la Coba, Stefan Duschek, and Gustavo A. Reyes del Paso. 2020. "Reliability, Factor Structure and Predictive Validity of the Widespread Pain Index and Symptom Severity Scales of the 2010 American College of Rheumatology Criteria of Fibromyalgia" Journal of Clinical Medicine 9, no. 8: 2460. https://doi.org/10.3390/jcm9082460
APA StyleGalvez-Sánchez, C. M., de la Coba, P., Duschek, S., & Reyes del Paso, G. A. (2020). Reliability, Factor Structure and Predictive Validity of the Widespread Pain Index and Symptom Severity Scales of the 2010 American College of Rheumatology Criteria of Fibromyalgia. Journal of Clinical Medicine, 9(8), 2460. https://doi.org/10.3390/jcm9082460