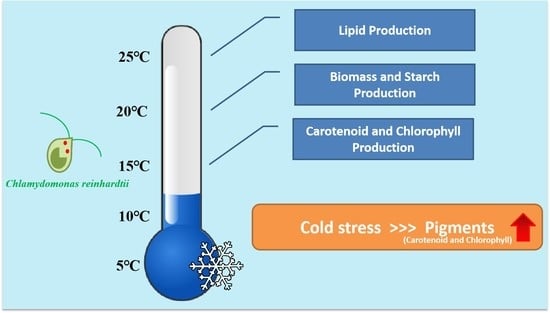
Pigment Production under Cold Stress in the Green Microalga Chlamydomonas reinhardtii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strains and Culture Conditions
2.2. Growth and Biomass Measurement
2.3. Photosynthetic Pigment Content
2.4. Starch and Lipid Production
2.5. Calculation of Productivities
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Hypothermal Stress on Growth of WT
3.2. Energy Storage Compounds of WT under Hypothermal Stress
3.3. Photosynthetic Pigment Accumulation of WT under Hypothermal Stress
3.4. Responses of the npq1lor1 Mutant to Cold Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanghera, G.S.; Wani, S.H.; Hussain, W.; Singh, N.B. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cruz, R.P.; Sperotto, R.A.; Cargnelutti, D.; Adamski, J.M.; de FreitasTerra, T.; Fett, J.P. Avoiding damage and achieving cold tolerance in rice plants. Food Energy Secur. 2013, 2, 96–119. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J. Cold stress signaling networks in Arabidopsis. J. Plant Biol. 2013, 56, 69–76. [Google Scholar] [CrossRef]
- Poiroux-Gonord, F.; Bidel, L.P.R.; Fanciullino, A.-L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health benefits of vitamins and secondary metabolites of fruits and vegetables and prospects to increase their concentrations by agronomic approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Carotenoids and other pigments as natural Colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrico, A.; Trupo, M.; Magarelli, R.; Balducchi, R.; Ferraro, A.; Hristoforou, E.; Marino, T.; Musmarra, D.; Casella, P.; Molino, A. Effectiveness of Dunaliella salina Extracts against Bacillus subtilis and Bacterial Plant Pathogens. Pathogens 2020, 28, 613. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.J.; Ben-Amotz, A. Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cisbeta-carotene production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Tumolo, T.; Lanfer-Marquez, U.M. Copper chlorophyllin: A food colorant with bioactive properties? Food Res. Int. 2012, 46, 451–459. [Google Scholar] [CrossRef]
- Perez-Galvez, A.; Viera, I.; Roca, M. Chemistry in the Bioactivity of Chlorophylls: An Overview. Curr. Med. Chem. 2017, 40, 4515–4536. [Google Scholar] [CrossRef] [Green Version]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their Derivatives Used in Food Industry and Medicine. Mini Rev. Med. Chem. 2017, 13, 1194–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.H. Chlamydomonas as a model organism. Annu. Rev. Plant Biol. 2001, 52, 363–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Reviewon the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef] [Green Version]
- Couso, I.; Vila, M.; Vigara, J.; Cordero, B.F.; Vargas, M.Á.; Rodríguez, H.; León, R. Synthesis of carotenoids and regulation of the carotenoid biosynthesis pathway in response to high light stress in the unicellular microalga Chlamydomonas reinhardtii. Eur. J. Phycol. 2012, 47, 223–232. [Google Scholar] [CrossRef]
- Fields, F.J.v.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Mayfield, S. Effects of the microalgae Chlamydomonas on gastrointestinal health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Murbach, T.S.; Glávits, R.; Endres, J.R.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A toxicological evaluation of Chlamydomonas reinhardtii, a green algae. Int. J. Toxicol. 2018, 37, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, R.; Gedi, M.A.; Eakpetch, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii Is a Potential Food Supplement with the Capacity to Out perform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Mıguez, F.; Holzinger, A.; Fernandez-Marin, B.; Garcıa-Plazaola, G.I.; Karsten, U.; Gustavs, L. Eco physiological changes and spore formation: Two strategies in response to low-temperature and high-light stress in Klebsormidium cf. flaccidum (Klebsormidiophyceae, Streptophyta). J. Phycol. 2020, 15, 649–661. [Google Scholar] [CrossRef]
- Soufi, S.; Rezgui, S.; Bettaeib, T. Early effects of chilling stress on the morphological and physiological status of pretreated Stevia rebaudiana Bert. seedlings. J. New Sci. 2015, 14, 467–472. [Google Scholar]
- Baroli, I.; Gutman, B.L.; Ledford, H.K.; Shin, J.W.; Chin, B.L.; Havaux, M.; Niyogi, K.K. Photo-oxidative Stress in a Xanthophyll-deficient Mutant of Chlamydomonas. J. Biol. Chem. 2003, 279, 6337–6344. [Google Scholar] [CrossRef] [Green Version]
- Niyogi, K.K.; Björkman, O.; Grossman, A.R. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 1997, 94, 14162–14167. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Sirikhachornkit, A.; Vuttipongchaikij, S.; Suttangkakul, A.; Yokthongwattana, K.; Juntawong, P.; Pokethitiyook, P.; Kangvansaichol, K.; Meetam, M. Increasing the Triacylglycerol Content in Dunaliella tertiolecta through Isolation of Starch-Deficient Mutants. J. Microbiol. Biotechnol. 2016, 28, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Anschau, A.; Caruso, C.S.; Kuhn, R.C.; Franco, T.T. Validation of the sulfo-phospho vanillin SPV method for the determination of lipid content in oleaginious microorganisms. Braz. J. Chem. 2017, 34, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Hempel, N.; Petrick, I.; Behrendt, F. Biomass productivity and productivity of fatty acids and amino acids of microalgae strains as key characteristics of suitability for biodiesel production. J. Appl. Phycol. 2012, 24, 1407–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valledor, L.; Furuhashi, T.; Hanak, A.M.; Weckwerth, W. Systemic cold stress adaptation of Chlamydomonas reinhardtii. Mol. Cell. Proteom. 2013, 12, 2032–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterwick, C.; Heaney, S.I.; Talling, J.F. Diversity in the influence of temperature on the growth rates of freshwater algae, and its ecological relevance. Fresh Biol. 2005, 50, 291–300. [Google Scholar] [CrossRef]
- Kudo, I.; Miyamoto, M.; Noiri, Y.; Maita, Y. Combined effects of temperature and iron on the growth and physiology of the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2000, 36, 1096–1102. [Google Scholar] [CrossRef]
- Xin, L.; Hong-ying, H.; Yu-ping, Z. Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour. Technol. 2011, 102, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Calderón, F.V.; López, J.S.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Ullmann, J. Current status of the algae production industry in Europe: An emerging sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Pankratz, S.; Oyedun, A.O.; Zhang, X.; Kumar, A. Algae pro-duction platforms for Canada’s northern climate. Renew. Sust. Energy Rev. 2017, 80, 109–120. [Google Scholar] [CrossRef]
- Pankratz, S.; Oyedun, A.O.; Kumar, A. Development of cost models of algae production in a cold climate using different production systems. Biofuels Bioprod. Biorefining 2019, 13, 1246–1260. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Z.; Zhu, S.; Zhou, W.; Dong, R.; Yuan, Z. Cultivation of Chlorella zofingiensis in bench-scale outdoor ponds by regulation of pH using dairy wastewater in winter, South China. Bioresour. Technol. 2012, 121, 76–82. [Google Scholar]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in micro-algae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Geider, R.; La-Roche, J. Redfield. revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Ali-zadeh, G. Low temperature stress increases Dunaliella cells population resistance to the effect of chronic dozes of UV-B radiation. CIB Tech. J. Biotechnol. 2012, 1, 36–39. [Google Scholar]
- Gilstad, M.; Johnsen, G.; Sakshaug, E. Photosynthetic parameters, pigment composition and respiration rates of the marine diatom Skeletonema costatum grown in continuous light and a 12/12 h light-dark cycle. J. Plankton Res. 1993, 15, 939–951. [Google Scholar] [CrossRef]
- Maikova, A.; Zalutskaya, Z.; Lapina, T.; Ermilova, E. The HSP70 chaperone machines of Chlamydomonas are induced by cold stress. J. Plant Physiol. 2016, 204, 85–91. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Juszczak, I.; Cvetkovic, J.; Zuther, E.; Hincha, D.K.; Baier, M. Natural Variation of Cold Deacclimation Correlates with Variation of Cold-Acclimation of the Plastid Antioxidant System in Arabidopsis thaliana Accessions. Front. Plant Sci. 2016, 7, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Kane, D.; Gill, V.; Boyd, P.; Burdon, R. Chilling oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta 1996, 198, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Chan, Z. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J. Integrat. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Zalutskaya, Z.; Skryabina, U.S.; Ermilova, E.V. Generation of hydrogen peroxide and transcriptional regulation of antioxidant enzyme expression in Chlamydomonas reinhardtii under hypothermia. Russ. J. Plant Physiol. 2019, 66, 223–230. [Google Scholar] [CrossRef]
- Ye, Z.W.; Jiang, J.G.; Wu, G.H. Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnol. Adv. 2008, 26, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Photoprotection revisited: Genetics and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Chua, E.T.; Dal’Molin, C.; Thomas-Hall, S.; Netzel, M.E.; Netzel, G.; Schenk, P.M. Cold and dark treatments induce omega-3 fatty acid and carotenoid production in Nannochloropsis oceanica. Algal Res. 2020, 51, 102059. [Google Scholar] [CrossRef]
- Ip, P.-F.; Chen, F. Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 2005, 40, 733–738. [Google Scholar] [CrossRef]
- Seel, W.; Baust, D.; Sons, D.; Albers, M.; Etzbach, L.; Fuss, J.; Lipski, A. Carotenoids are used as regulators for membrane fluidity by Staphylococcus xylosus. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zakar, T.; Herman, E.; Vajravel, S.; Kovacs, L.; Knoppová, J.; Komenda, J.; Domonkos, I.; Kis, M.; Gombos, Z.; Laczko-Dobos, H. Lipid and carotenoid cooperation-driven adaptation to light and temperature stress in Synechocystis sp. PCC6803. Biochim. Biophys. Acta 2017, 1858, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Pogson, B.; McDonald, K.A.; Truong, M.; Britton, G.; DellaPenna, D. Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell. 1996, 8, 1627–1639. [Google Scholar] [PubMed] [Green Version]
- Alboresi, A.; Dall’Osto, L.; Aprile, A.; Carillo, P.; Roncaglia, E.; Cattivelli, L.; Bassi, R. Reactive oxygen species and transcript analysis upon excess light treatment in wild-type Arabidopsis thaliana vs a photosensitive mutant lacking zeaxanthin and lutein. BMC Plant Biol. 2011, 11, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyogi, K.K.; Shih, C.; Chow, W.S.; Pogson, B.J.; DellaPenna, D.; Björkman, O. Photoprotection in a zeaxanthin-and lutein-deficient double mutant of Arabidopsis. Photosynth. Res. 2001, 67, 139–145. [Google Scholar] [CrossRef] [PubMed]





| Productivity | |||||
|---|---|---|---|---|---|
| 5 °C | 10 °C | 15 °C | 20 °C | 25 °C | |
| Biomass (g L−1 day−1) | 1.23 ± 0.08 * | 1.75 ± 0.11 * | 1.76 ± 0.14 * | 2.01 ± 0.09 * | 1.95 ± 0.06 |
| Starch (mg L−1 day−1) | 54.97 ± 2.15 * | 94.59 ± 3.27 | 124.24 ± 4.21 * | 135.00 ± 5.62 * | 100.00 ± 4.43 |
| Lipid (mg L−1 day−1) | 163.73 ± 4.28 * | 499.98 ± 3.76 * | 256.38 ± 2.54 * | 871.58 ± 5.94 * | 1016.99 ± 4.11 |
| Chlorophyll (mg L−1 day−1) | 31.79 ± 1.15 * | 43.39 ± 0.97 | 55.18 ± 2.01 * | 48.83 ± 1.96 * | 40.37 ± 1.41 |
| Carotenoids (mg L−1 day−1) | 7.09 ± 0.19 | 9.80 ± 0.51 * | 13.48 ± 0.32 * | 10.55 ± 0.08 * | 6.69 ± 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potijun, S.; Yaisamlee, C.; Sirikhachornkit, A. Pigment Production under Cold Stress in the Green Microalga Chlamydomonas reinhardtii. Agriculture 2021, 11, 564. https://doi.org/10.3390/agriculture11060564
Potijun S, Yaisamlee C, Sirikhachornkit A. Pigment Production under Cold Stress in the Green Microalga Chlamydomonas reinhardtii. Agriculture. 2021; 11(6):564. https://doi.org/10.3390/agriculture11060564
Chicago/Turabian StylePotijun, Supakorn, Chonlada Yaisamlee, and Anchalee Sirikhachornkit. 2021. "Pigment Production under Cold Stress in the Green Microalga Chlamydomonas reinhardtii" Agriculture 11, no. 6: 564. https://doi.org/10.3390/agriculture11060564
APA StylePotijun, S., Yaisamlee, C., & Sirikhachornkit, A. (2021). Pigment Production under Cold Stress in the Green Microalga Chlamydomonas reinhardtii. Agriculture, 11(6), 564. https://doi.org/10.3390/agriculture11060564