1. Introduction
A vacuum is a region from which air and other gases have been removed and with a pressure much less than the surface pressure on earth. The amount removed depends on the requirements of the study or application and is limited by the technology used. Many scientists work with samples in vacuum systems. The reasons for this are several-fold: first, many samples react with gases at atmospheric pressure, which means that they must be kept in a clean environment under vacuum conditions; second, many of the experimental techniques (spectroscopies, microscopies and diffraction techniques) used to measure sample properties need vacuum conditions to operate and furthermore, the ability to establish a vacuum also allows for the chamber to then be filled with the gases required to simulate a desired planetary atmosphere. In this context, fully equipped and monitored planetary-simulation facilities enable a broad range of tests with biological, geological and chemical materials. Of particular interest for such applications is the ability to define and control conditions such as vacuum level, gas composition, low and high temperatures, and UV irradiation.
The modest knowledge that we possess concerning planetary environments has been garnered primarily from space missions, which are highly demanding in both time and cost. Because of the obvious technical and economical limitations on
in situ planetary exploration, laboratory simulations are one of the most feasible research options to make advances both in planetary science and in developing a consistent description of the origin of life. Therefore, the most general question of astrobiology concerns the understanding of life and the processes that lead to its origin, evolution and distribution, on Earth and elsewhere, as part of the evolution of the cosmos. Laboratory-based facilities are able to mimic the conditions found in the atmospheres and on the surfaces of the majority of planetary objects (e.g., Mars, Titan, Europa). Planetary-simulation chambers have experienced extraordinary improvement from the first methodological models and instrumental designs of the 1960s to the present [
1,
2,
3,
4,
5,
6,
7,
8]. They have become extremely useful tools for assessing analytical protocols for instruments and sensors that operate outside the Earth’s environment and for emulating various experiments that involve biological, geological and physico-chemical issues [
9,
10,
11,
12,
13,
14,
15].
The Planetary Atmosphere and Surfaces Chamber (PASC) [
8] is a versatile planetary-simulation chamber that is capable of producing environments with computer-controlled gas compositions, pressure in the atmosphere and sample temperatures that can be representative of most of the planets in the Solar System. This equipment was specifically developed to make feasible the
in situ irradiation and physico-chemical characterization of samples. The PASC simulation chamber was developed to be a useful tool in several scientific and technological areas of knowledge, such as engineering, chemistry, geology and biology. PASC has produced relevant scientific outcomes related to astrobiology and habitability research: The UV-absorbing properties of basaltic dust (Martian analog) have been evaluated as a function of its thickness and mass [
9]. Stability of saline water under Mars conditions have been studied; indicating that salty locations might allocate water to be periodically and locally liquid on Mars [
12]. Furthermore, the survival capability of specific bacteria exposed to simulated Mars conditions has been recently confirmed [
14], as have the high resistance and survival capability of eukaryotic symbiotic organisms (lichen) under simulated Mars and space conditions [
15]. Furthermore, the photostability or molecular damage of nucleobases and peptide biomolecules under UV irradiation has also been investigated [
11,
13]. These studies confirm the significance of simulation-chamber facilities as an emerging type of instrumentation for the emulation of planetary conditions and the assessment of various multidisciplinary, astrobiologically relevant scenarios (
Figure 1).
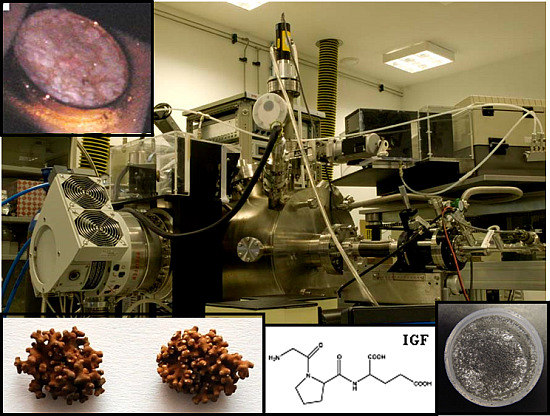
Figure 1.
Astrobiological applications of the Planetary Atmosphere and Surfaces Chamber (PASC): Mars minerals show deliquescence process that may occur on Mars; Prebiotic chemistry show biomolecule adsorption on a pyrite surface; Habitability show lichens due to their survival capacity; Photochemistry shows a peptide molecule susceptible to make this physical phenomenon and Sensors shows basaltic dust to study its UV transmittance.
Figure 1.
Astrobiological applications of the Planetary Atmosphere and Surfaces Chamber (PASC): Mars minerals show deliquescence process that may occur on Mars; Prebiotic chemistry show biomolecule adsorption on a pyrite surface; Habitability show lichens due to their survival capacity; Photochemistry shows a peptide molecule susceptible to make this physical phenomenon and Sensors shows basaltic dust to study its UV transmittance.
2. Results and Discussion
2.1. Technological Applications: Sensors for Planetary Exploration
Measurements of the transmittance of Martian dust, as a function of depth, serve to estimate the protection from UV irradiation provided by the Mars regolith, which can shield microorganisms from the damaging effects of UV fluxes. Furthermore, dust will inevitably be deposited on any instrumentation sent to the surface of Mars, and the calibration of any UV sensors for future Mars missions should take the effect of such dust deposition into account.
In this context, NASA’s Mars Exploration Rovers have indicated the presence of magnetite and olivine on Mars; this finding indicates that the dust is of basaltic origin [
16]. Basalt is therefore one of the primary components of the dust on the surface of Mars, and consequently, the transmittance of basalt dust in the near-UV range was investigated in PASC. The Martian regolith was exposed to solar irradiation in the near-UV range (200–390 nm). The near-UV irradiation of the basalt dust on Mars was experimentally simulated to determine the transmittance of the dust as a function of its mass and thickness. These data can aid in quantifying the absorption of the dust deposited on sensors intended to measure the UV intensity on the surface of Mars. The minimum thickness of the dust that would correspond to near-zero transmittance in the near-UV range was measured. Hypothetical Martian microorganisms living in the deeper layers of the dusty regolith would be protected from damaging solar UV irradiation.
The reduction of the UV transmittance as a function of the mass and thickness of the basalt-dust deposition was calibrated [
9]. It was found that the UV transmittance value drops to nearly zero for a basalt-dust thickness of ~300 µm, and therefore, microorganisms living at deeper layers than this would be protected from damaging UV irradiation on Mars. The estimated surface coverage that is produced through the settling of Martian dust suspended in the atmosphere is Sd/S = 0.0036 per day,
i.e., 0.36% of a surface becomes covered in one day, for particles of ~5.5 µm in diameter (the upper and lower limits are 0.03% and 2.9%); see [
17]. These values can be used to determine the decrease in the transmittance as a function of time during quiet periods, when no wind events or dust storms are occurring. In only the most optimistic case, in which 0.03% of an area becomes covered by dust in one day, would such dust settling have little effect on the measurement of the UV flux on the surface of Mars for missions of a few months in duration; moreover, even in that case, occasional dust storms would necessitate the use of some device for dust removal.
2.2. Geological Applications: Mars Minerals
An essential objective of the exploration of Mars was to determine whether liquid water exists there today or existed in the past [
18]. It has been hypothesized that the presence of salts may depress the freezing point and allows liquid saline water to be sporadically stable on the Martian surface [
19,
20]. Chloride salts have been found on the Meridiani and Gusev sites and in widespread deposits in the southern Martian hemisphere. Furthermore, the Phoenix lander found perchlorate salts (mainly magnesium and sodium perchlorate) on Mars’ arctic soil. The existence of these salts on the surface of Mars is significant due to their capability to lower the freezing point of water, and also because they can absorb water vapor until they melt and form a liquid solution (deliquesce process).
Thus, the stability of sodium perchlorate under Martian atmospheric conditions has been experimentally investigated in the Planetary Atmosphere and Surfaces Chamber (PASC). A sample of sodium perchlorate was placed on the PASC cold finger, and visible-wavelength imaging and infrared spectroscopy were used to study the hydration level and phase changes of the sample. It was experimentally demonstrated that under Martian atmospheric conditions (CO
2 at 700 Pa), small amounts of sodium perchlorate (~1 mg) and mixtures of water ice and sodium perchlorate melt into liquid at this temperature 225 K; spontaneously they absorb moisture until they melt into a liquid solution. These results indicate that salty environments cause liquid water to be locally and sporadically stable on present-day Mars. Therefore, PASC has been used to simulate the deliquescence process that may occur on Mars [
12].
2.3. Chemical Applications: UV Processes in Biomolecules
The organic compounds that are observed in the interstellar medium and in Solar System bodies are of particular interest for revealing the chemistry that may elucidate the origin of life [
21,
22]. Even if these molecules can be synthesized under space conditions, the important question of whether these molecules are stable under irradiation remains. The photostability and photochemical processes of these molecules have therefore attracted considerable interest, and this question has been discussed as a possible evolutionary factor in the emergence of life. In this context, the photostability or molecular damage of nucleobases and peptide biomolecules under UV irradiation has been investigated.
Biomolecular photostability depends on the molecular environment (gas phase, solid matrix, surfaces,
etc.). Peeters
et al. [
23] have reported that in an argon matrix (frozen substrate), the energy absorbed by a molecule can be easily dissipated, thereby lowering the photodestruction efficiency compared to the same molecule in the gas phase. With the objective of investigating the stability and/or reactivity of biomolecules under exposure to UV, the influence of UV irradiation on peptides isolated in an argon matrix has been studied as a means of testing their stability under UV irradiation. The successful formation of a tripeptide (IGF) argon matrix has been observed under vacuum inside the PASC chamber, followed by the
in situ UV irradiation of the molecular matrix and the
in situ characterization of its reactivity after UV-irradiation [
11]. The spectroscopic combination of Reflexion Absorption Infrared Spectroscopy (RAIRS) and X-ray Photoelectron Spectroscopy (XPS) results provided evidence that the UV irradiation of peptides induces a chemical reaction that causes the partial conversion of amide tautomer into an imidic acid tautomer (
Figure 2). A very important conclusion that can be drawn from these findings is that peptidic bonds, which lie at the heart of all biomolecules, are fragile under UV irradiation. Changing the structure or chemical functionalities of biomolecules is likely to affect their biological activity in a dramatic way; however, little is known about the stability of these molecules under UV irradiation, conditions which may be encountered in various natural environments. Therefore, the next challenge is to study these processes and gain an understanding of their chemistry using vacuum chambers as an experimental platform.
Furthermore, to date, very little attention has been paid to the role of the surface in photochemical processes; as opposed to studying molecules in the gas phase or within a matrix, as described above, surfaces mimic a more realistic environment for prebiotic chemistry. Therefore, the UV irradiation of adenine on various surfaces (silicon and gold) has been performed inside the PASC to study the photostability and photochemistry of this nucleic base [
13].
A spectroscopic study using XPS and RAIRS has demonstrated that UV irradiation promotes a slight reorientation of the molecules in addition to desorption on both gold and silicon surfaces and partial dissociation only on the gold surface therefore, the photostability process depends on the nature of the surface, and adenine molecules present a lower desorption rate on silicon (where no fragmentation is evident) than on gold. A very relevant implication of this result is that condritic oxide particles are more appropriate than metallic particles for catalyzing reactions and delivering nucleobases in space because nucleobases and other similar chemical forms will fragment easily when adsorbed on metallic grains.
Figure 2.
Testing peptides Ar+ matrix stability under UV irradiation, which induces a chemical tautomeric transformation.
Figure 2.
Testing peptides Ar+ matrix stability under UV irradiation, which induces a chemical tautomeric transformation.
The XPS and Infrared Spectroscopy techniques offer complementary information and are highly sensitive and non-destructive, and then they well suited for characterizing the possible chemical changes in molecules due to UV irradiation. Therefore, the spectroscopic analysis of biomolecules (nucleic bases and peptides) on surfaces or in an argon matrix, in combination with the experimental setup (UV irradiation in ultra-high-vacuum conditions) of the planetary atmospheres simulation chamber, offers a novel approach to understanding certain chemical processes related to the origin of life and precursor molecules.
2.4. Physico-Chemical Applications: Prebiotic Chemistry
The interactions of biomolecules on mineral surfaces are attracting increasing interest with regard to various aspects of the chemistry and physics of mineral surfaces, mainly in prebiotic chemistry research [
24,
25]. In this context, a “catalytic effect” of mineral surfaces is invoked to explain prebiotic polymer formation on the early Earth. These mineral surfaces have the potential to facilitate prebiotic polymerization; minerals might adsorb and concentrate biomolecules and may catalyze reactions. However, the precise nature of the interactions between the mineral host and the biomolecule guest has to be studied in detail.
Amino acids are the building blocks of proteins, and they are the essential units from which highly ordered molecular networks and more complex molecules are built up through polymerization reactions [
26,
27]. Therefore, in this new context, we can include the interactions of simple molecules as amino acids on catalytic surfaces, which may play a crucial role in the origin of life and the field of prebiotic chemistry. It is well known that the adsorption of organic molecules or biopolymers on a mineral surface may substantially modify the morphology, surface composition and reactivity of the mineral, thereby affecting its tailoring properties. An exhaustive assessment, at the molecular level, is now essential to investigate the chemical reactions involved and to address the role of mineral surfaces as well as other chemical and geophysical prebiotic conditions in the formation of peptides from amino acids. Furthermore, surface science techniques are very promising tools for studying these molecular processes on catalytic mineral surfaces to obtain molecular information by applying a broad range of spectroscopy and microscopy techniques. Thus, the simulation of prebiotic chemistry reactions based on the complementary use of surface science techniques and well-controlled conditions in a vacuum simulation chamber promises to be a fruitful investigative approach. An experiment using this strategy to evaluate how different experimental conditions modify molecular interactions with catalytic surfaces and to shed some light on the “polymerization on the rocks” [
28,
29] hypothesis is already under design.
2.5. Biological Applications: Survival of Microorganisms
At present, there is considerable interest in microorganisms that may be capable of developing mechanisms to adapt to and survive harsh planetary and space conditions [
30,
31]. Extremophile organisms could serve as good model systems for understanding the interplanetary transfer of photosynthetic organisms; they could be suitable candidates for survival in space and under Martian conditions because of their optimal latency characteristics. Simulation chambers are essential tools for the assessment of the survival/adaptation of biological organisms under the harsh conditions of other planets [
14,
15].
As an experimental case, studies of the survival capacity of lichens (eukaryotic and prokaryotic symbiotic organisms) under the conditions that prevail on the surface of Mars have been performed in PASC. After 120 h of exposure to simulated but representative Martian atmospheric, temperature, pressure and UV conditions, the photosynthetic performance of the lichen photobiont was unaltered, demonstrating its high resistance to these harsh conditions [
15]. Because PASC is a unique instrument that is capable of simulating a wide variety of environmental conditions, it effectively facilitated the study of the high resistance of this specific lichen species to the harsh conditions on Mars [
15]. Additionally, an acidophilic chemolithotroph isolated from Río Tinto and
Deinococcus radiodurans microorganisms were exposed to simulated Mars environmental conditions in PASC under the protection of a layer of Mars regolith analogue. It was concluded that the presence of a thin layer of Mars regolith is critical to significantly reduce radiation doses and provide a shielding layer for microorganisms. Habitability increases considerably under only a few millimeters of regolith protection [
14].
Therefore, planetary-simulation chambers are an ideal and an accurate tool for contributing to future space missions and for the design of successful scientific experiments in planetary environments to be performed on real planetary bodies (in situ missions). Our simulation chamber will contribute with complementary knowledge concerning the survival and adaptation of extremophile organisms under the extreme planetary conditions in which these organisms live and to which they are adapted. Further astrobiological experiments using lichens should investigate reactivation procedures under Martian atmospheric conditions to confirm the lichens’ ability to live in such environments. Studies of extremophile organisms expand the powerful capabilities of this planetary-environment simulation chamber.
3. Experimental Section
PASC (Planetary Atmospheres and Surfaces Chamber) is an ultra-high-vacuum (UHV) simulation chamber 500 mm long by 400 mm diameter (total volume of about 62,800 cm
3) that is capable of reproducing atmospheric compositions and surface temperatures that are representative of most planetary objects. This equipment was specifically developed to make feasible the
in situ UV irradiation and infrared spectroscopy characterization of samples under study (see the details provided in ref. [
8],
Figure 3). The total pressure range of the chamber is from 7 mbar to 5 × 10
−9 mbar, and the partial pressures of the gases in the chamber can be set with this precision. To simulate a particular atmosphere, the gases are mixed in a many-fold to the required proportions, and the gas composition is constantly monitored by a residual gas analyzer mass spectrometer. The temperature of the sample is regulated by a commercial helium cooling system connected to the sample holder, and it can be adjusted from −269 °C to 52 °C. Sample size ranges are from 5 to 35 mm diameter; however other sizes of sample container are available for specific developments. Various irradiation sources are available to mimic electron (5 KeV), ion (5 KeV) and UV irradiation (200–500 nm).
In summary, the range of parameters that can be simulated on the chamber are:
- -
Total pressure ranges from 7 mbar to 5 × 10−9 mbar. Partial pressure of the gasses can be set with this precision; and it is regulated via a residual gas analyzer with ca ppm precision;
- -
Temperature ranges from −269 °C to 52 °C;
- -
Sample size ranges from 5 to 35 mm diameter (other sizes of sample container are available for specific developments);
- -
Available irradiation sources: up to 5 KV-ions (ions) 5 KV-electrons and Deuterium UV lamp (200–500 nm).
Figure 3.
Photograph of the Planetary Atmosphere and Surfaces Chamber (PASC), planetary-simulation equipment.
Figure 3.
Photograph of the Planetary Atmosphere and Surfaces Chamber (PASC), planetary-simulation equipment.
4. Conclusions
Multiple studies in many scientific and technological fields have confirmed the importance of simulation-chamber facilities as promising instrumentation for the emulation of planetary conditions and the assessment of multidisciplinary, astrobiologically relevant scenarios. The relevance of simulation-chamber facilities as essential instrumentation for the emulation of planetary conditions and, therefore, for the accrual of knowledge regarding several interdisciplinary astrobiological topics has been demonstrated.
Future planetary and space mission will be driven by innovative technology and challenging scientific experiments, the success of which will be ensured through preliminary tests in laboratory facilities. In this context, simulation chambers will play a crucial role in garnering complementary knowledge and exploring potential difficulties before such space missions are designed and launched.