Development of Highly pH-Sensitive Hybrid Membranes by Simultaneous Electrospinning of Amphiphilic Nanofibers Reinforced with Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
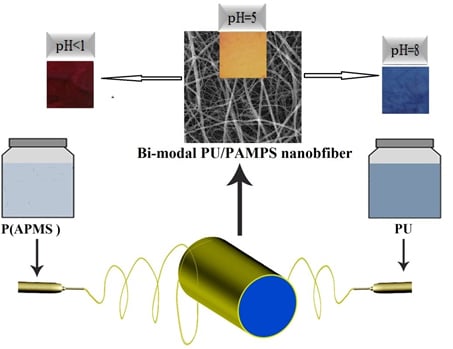
2.2. Preparation of Electrospinning Solutions
2.3. Electrospinning
2.4. Membrane Characterization
3. Results and Discussion
3.1. Morphology of Nanofibers
3.1.1. Effect of Hydrophilic Moieties (PAMPS) on Fiber Morphology
3.1.2. Effect of GO on Morphology
3.2. Halochromic Behavior of ENMs with Different Polymer Ratios
3.2.1. Effect of the Hydrophilic Moiety (PAMPS) on Sensing Time
3.2.2. pH Sensing Behaviour of Reference Dye System and ENMs
3.3. Halochromic Behavior of ENMs with Different GO Ratios
3.3.1. Effect of GO on Sensing Time
3.3.2. pH Sensing Behavior of Reference Dye System and ENMs with Incorporated GO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flam, E. Method of Monitoring the Condition of the Skin or Wound. US Patent 5181905 A, 26 January 1993. [Google Scholar]
- Tassanawat, S.; Phandee, A.; Magaraphan, R.; Nithitanakul, M.; Manuspiya, H. pH-sensitive PP/clay nanocomposites for beverage smart packaging. In Proceedings of the 2nd Conference on Nano/Micro IEEE International Engineered and Molecular Systems, Bangkok, Thailand, 16–19 January 2007; pp. 478–482. [Google Scholar]
- Steyaert, I.; Vancoillie, G.; Hoogenboom, R.; De Clerck, K. Dye immobilization in halochromic nanofibers through blend electrospinning of a dye-containing copolymer and polyamide-6. Polym. Chem. 2015, 6, 2685–2694. [Google Scholar] [CrossRef]
- Golmohammadi Rostami, S.; Sorayani Bafqi, M.S.; Bagherzadeh, R.; Latifi, M.; Gorji, M. Multi-layer electrospun nanofiber mats with chemical agent sensor function. J. Ind. Text. 2015, 45, 467–480. [Google Scholar] [CrossRef]
- Razaq, S.; Wilkins, R.J.; Urban, J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J. 2003, 12, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, E.R.; Kirkpatrick, P.R.; Thompson, R.C. The effect of environmental pH on glycosaminoglycan metabolism by normal human chondrocytes. J. Lab. Clin. Med. 1976, 87, 198–205. [Google Scholar]
- Waldman, S.D.; Couto, D.C.; Omelon, S.J.; Kandel, R.A. Effect of sodium bicarbonate on extracellular pH, matrix accumulation, and morphology of cultured articular chondrocytes. Tissue Eng. 2004, 10, 1633–1640. [Google Scholar] [CrossRef]
- Masuda, T.; Maruyama, H.; Arai, F.; Anada, T.; Fukuda, T.; Suzuki, O. Development of a pH indicator immobilized-gel-sheet for microenviroment analysis. In Proceedings of the 2009 International Symposium on Micro-NanoMechatronics and Human Science IEEE, Nagoya, Japan, 9–11 November 2009; pp. 362–367. [Google Scholar]
- Nakao, M.; Inoue, S.; Yoshinobu, T.; Iwasaki, H. High-resolution pH imaging sensor for microscopic observation of microorganisms. Sens. Actuators B 1996, 34, 234–239. [Google Scholar] [CrossRef]
- Van der Schueren, L.; Mollet, T.; Ceylan, Ö.; De Clerck, K. The development of polyamide 6.6 nanofibers with a pH-sensitive function by electrospinning. Eur. Polym. J. 2010, 46, 2229–2239. [Google Scholar] [CrossRef]
- Agarwal, A.; Raheja, A.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B 2012, 161, 1097–1101. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Meyer, T.; Steyaert, I.; Ceylan, Ö.; Hemelsoet, K.; Van Speybroeck, V.; De Clerck, K. Polycaprolactone and polycaprolactone/chitosan nanofibres functionalized with the pH-sensitive dye nitrazine yellow. Carbohydr. Polym. 2013, 91, 284–293. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Ghazawy, R.A.; Farag, R.K.; Elsaeed, S.M. Synthesis and characterization of pH-sensitive PAMPS/PVP nanogels in aqueous media. Polym. Adv. Technol. 2011, 22, 732–737. [Google Scholar] [CrossRef]
- Peterson, D. pH-Sensitive Hydrogel. In Encyclopedia of Microfluidics and Nanofluidics; Springer: Boston, MA, USA, 2011; pp. 1–5. [Google Scholar]
- Amini, G.; Samiee, S.; Gharehaghaji, A.A.; Hajiani, F. Fabrication of polyurethane and nylon 66 hybrid electrospun nanofiber layer for waterproof clothing applications. Adv. Polymer Technol. 2016, 35, 419–427. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, Z.; Li, X. Improving filtration performance of electrospun nanofiber mats by a bimodal method. J. Appl. Polym. Sci. 2013, 128, 1089–1094. [Google Scholar] [CrossRef]
- Jung, K.; Pourdeyhimi, B.; Zhang, X. Synthesis and characterization of polymer-filled nonwoven membranes. J. Appl. Polym. Sci. 2011, 119, 2568–2575. [Google Scholar] [CrossRef]
- Chen, H.; Rahmathullah, A.M.; Palmese, G.R.; Elabd, Y.A. Polymer-polymer nanocomposite membranes as breathable barriers with electro-sensitive permeability. In ACS Symposium Series; Oxford University Press: Oxford, UK, 2009; Volume 1016, pp. 307–322. [Google Scholar]
- Bai, H.; Li, C.; Wang, X.; Shi, G. A pH-sensitive graphene oxide composite hydrogel. Chem. Comm. 2010, 46, 2376–2378. [Google Scholar] [CrossRef]
- Kim, S.J.; Lim, J.Y.; Kim, I.Y.; Lee, S.H.; Lee, T.S.; Kim, S.I. Optimum parameters for production of nanofibres based on poly (2-acrylamido2-methyl-1-propane sulfonic acid) by electro-spinning. Smart Mater. Struct. 2005, 14, 16–20. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, K.M.; Kim, S.I. The effect of electric current on the processing of nanofibres formed from poly(2-acrylamido-2-methyl-1-propane sulfonic acid). Scripta Mater. 2004, 51, 31–35. [Google Scholar]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf. A 2001, 187, 469–481. [Google Scholar] [CrossRef]
- Lee, S.; Obendorf, S.K. Barrier effectiveness and thermal comfort of protective clothing materials. J. Text. Inst. 2007, 98, 87–98. [Google Scholar] [CrossRef]
- Lee, S.; Obendorf, S.K. Statistical model of pesticide penetration through woven work clothing fabrics. Arch. Environ. Contam. Toxicol. 2005, 49, 266–273. [Google Scholar] [CrossRef]
- Gorji, M.; Jeddi, A.; Gharehaghaji, A. Fabrication and characterization of polyurethane electrospun nanofiber membranes for protective clothing applications. J. Appl. Polym. Sci. 2012, 125, 4135–4141. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, W.; Jiang, Z.; Amarjargal, A.; Park, C.H.; Pant, H.R.; Kim, C.S. Two-nozzle electrospinning of (MWNT/PU)/PU nanofibrous composite mat with improved mechanical and thermal properties. Current Appl. Phys. 2013, 13, 1247–1255. [Google Scholar] [CrossRef]
- Gorji, M.; Bagherzadeh, R.; Fashandi, H. Electrospun nanofibers in protective clothing. In Electrospun Nanofibers; Woodhead Publishing: Cambridge, UK, 2017; pp. 571–598. [Google Scholar]
- Gorji, M.; Sadeghian Maryan, A. Breathable-windproof membrane via simultaneous electrospinning of PU and P (AMPS-GO) hybrid nanofiber: Modeling and optimization with response surface methodology. J. Ind. Text. 2018, 47, 1645–1663. [Google Scholar] [CrossRef]
- Lee, S.; Obendorf, S.K. Transport properties of layered fabric systems based on electrospun nanofibers. Fibers Polym. 2007, 8, 501–506. [Google Scholar] [CrossRef]
- Shemshadi, R.; Ghafarian, R.; Gorji, M.; Avazverdi, E. A smart thermoregulatory nanocomposite membrane with improved thermal properties: Simultaneous use of graphene family and micro-encapsulated phase change material. Text. Res. J. 2018. [Google Scholar] [CrossRef]
- Pant, H.R.; Park, C.H.; Tijing, L.D.; Amarjargal, A.; Lee, D.H.; Kim, C.S. Bimodal fiber diameter distributed graphene oxide/nylone-6 composite nanofibrous mats via electrospinning. Colloids Surf. A 2012, 407, 121–125. [Google Scholar] [CrossRef]
- Penner, M.H. Ultraviolet, Visible, and Fluorescence Spectroscopy; Nielsen, S.S., Ed.; Food Analysis; Springer: Boston, MA, USA, 2014; Chapter 22; p. 389. [Google Scholar]
- Schoolaert, E.; Steyaert, I.; Vancoillie, G.; Geltmeyer, J.; Lava, K.; Hoogenboom, R.; De Clerck, K. Blend electrospinning of dye-functionalized chitosan and poly (ε-caprolactone): Towards biocompatible pH-sensors. J. Mater. Chem. B 2016, 4, 4507–4516. [Google Scholar] [CrossRef]
- Gorji, M.; Karimi, M.; Nasheroahkam, S. Electrospun PU/P (AMPS-GO) nanofibrous membrane with dual-mode hydrophobic–hydrophilic properties for protective clothing applications. J. Ind. Text. 2018, 47, 1166–1184. [Google Scholar] [CrossRef]











| pH-Indicator Dye | pH Range | Color Change |
|---|---|---|
| Bromophenol blue | (Y) 3.0–4.6 (BV) | yellow to purple |
| Bromocresol green | (Y) 3.8–5.4 (B) | yellow to blue |
| Methyl red | (R) 4.2–6.2 (Y) | red to yellow |
| Bromophenol red | (Y) 5.2–6.8 (R) | yellow to red |
| Sample Code | PU/PAMPS | GO (w/w %) |
|---|---|---|
| 100 | 100/0 | 0 |
| 80 | 80/20 | 0 |
| 60 | 60/40 | 0 |
| 40 | 40/60 | 0 |
| 40/1 | 40/60 | 1 |
| 40/2 | 40/60 | 2 |
| 40/3 | 40/60 | 3 |
| 40/4 | 40/60 | 4 |
| Sample Code | PU/PAMPS | Solution Response Time (s) | Vapor Response Time (s) |
|---|---|---|---|
| 100 | 100/0 | 80 | 300 |
| 80 | 80/20 | 40 | 170 |
| 60 | 60/40 | 30 | 100 |
| 40 | 40/60 | 20 | 60 |
| pH = 2 | pH = 3 | pH = 4 | pH = 5 | pH = 6 | pH = 7 | pH = 8 | |
|---|---|---|---|---|---|---|---|
| pH < 1 | 23.94 | 26.04 | 50.94 | 66.71 | 69.89 | 64.1 | 47.37 |
| pH = 2 | 8.77 | 45.98 | 68.62 | 81.05 | 86.34 | 67.36 | |
| pH = 3 | 40.46 | 63.26 | 76.49 | 84.82 | 66.8 | ||
| pH = 4 | 25.63 | 49.59 | 82.93 | 61.21 | |||
| pH = 5 | 30.1 | 76.71 | 57.46 | ||||
| pH = 6 | 53.11 | 41.47 | |||||
| pH = 7 | 29.68 |
| pH = 2 | pH = 3 | pH = 4 | pH = 5 | pH = 6 | pH = 7 | pH = 8 | |
|---|---|---|---|---|---|---|---|
| pH < 1 | 28.37 | 25.14 | 47.58 | 64.83 | 36.82 | 53.45 | 57.15 |
| pH = 2 | 11.87 | 37.54 | 50.73 | 46.27 | 72.62 | 78.06 | |
| pH = 3 | 27.86 | 43.49 | 34.7 | 61.94 | 69.84 | ||
| pH = 4 | 18.84 | 28.91 | 58.22 | 74.71 | |||
| pH = 5 | 44.58 | 72.04 | 92.05 | ||||
| pH = 6 | 30.08 | 51.83 | |||||
| pH = 7 | 38.61 |
| Sample Code | Solution Response Time (s) | Vapor Response Time (s) |
|---|---|---|
| 40/1 | 5 | 15 |
| 40/2 | 3 | 12 |
| 40/3 | 0 | 9 |
| 40/4 | 0 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorji, M.; Sadeghianmaryan, A.; Rajabinejad, H.; Nasherolahkam, S.; Chen, X. Development of Highly pH-Sensitive Hybrid Membranes by Simultaneous Electrospinning of Amphiphilic Nanofibers Reinforced with Graphene Oxide. J. Funct. Biomater. 2019, 10, 23. https://doi.org/10.3390/jfb10020023
Gorji M, Sadeghianmaryan A, Rajabinejad H, Nasherolahkam S, Chen X. Development of Highly pH-Sensitive Hybrid Membranes by Simultaneous Electrospinning of Amphiphilic Nanofibers Reinforced with Graphene Oxide. Journal of Functional Biomaterials. 2019; 10(2):23. https://doi.org/10.3390/jfb10020023
Chicago/Turabian StyleGorji, Mohsen, Ali Sadeghianmaryan, Hossein Rajabinejad, Saman Nasherolahkam, and Xiongbiao Chen. 2019. "Development of Highly pH-Sensitive Hybrid Membranes by Simultaneous Electrospinning of Amphiphilic Nanofibers Reinforced with Graphene Oxide" Journal of Functional Biomaterials 10, no. 2: 23. https://doi.org/10.3390/jfb10020023
APA StyleGorji, M., Sadeghianmaryan, A., Rajabinejad, H., Nasherolahkam, S., & Chen, X. (2019). Development of Highly pH-Sensitive Hybrid Membranes by Simultaneous Electrospinning of Amphiphilic Nanofibers Reinforced with Graphene Oxide. Journal of Functional Biomaterials, 10(2), 23. https://doi.org/10.3390/jfb10020023