Icariin: A Promising Natural Product in Biomedicine and Tissue Engineering
Abstract
:1. Introduction
2. Botanical Origins and Distribution
3. Traditional Applications and Ethnopharmacology of Epimedium Species
4. Physicochemical Properties
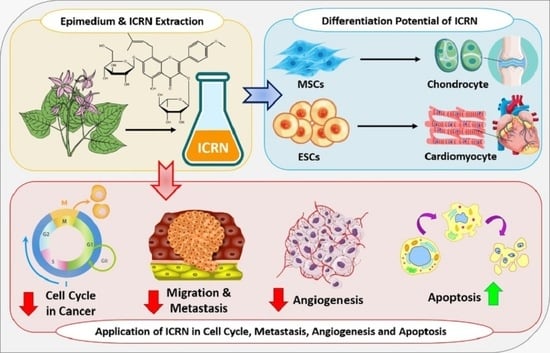
5. Bioengineering Application of ICRN
5.1. Bone Tissue Engineering
5.2. Cartilage Tissue Engineering
6. Cancer Therapy by ICRN
6.1. Apoptosis Induction
6.2. Inhibition of Cancer Cell Proliferation
6.3. Angiogenesis Inhibition
6.4. Metastasis and Migration Inhibition
6.5. Regulation of Immune System
7. Effects of ICRN on Drugs Used in Cancer Therapy
7.1. Multidrug Resistance (MDR) Inhibition
7.2. Effects of ICRN on Cisplatin
8. Other pharmacological Effects of ICRN
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mobaraki, F.; Momeni, M.; Yazdi, M.E.T.; Meshkat, Z.; Toosi, M.S.; Hosseini, S.M. Plant-derived synthesis and characterization of gold nanoparticles: Investigation of its antioxidant and anticancer activity against human testicular embryonic carcinoma stem cells. Process. Biochem. 2021, 111, 167–177. [Google Scholar] [CrossRef]
- Yazdi, M.E.; Amiri, M.S.; Darroudi, M. Biopolymers in the Synthesis of Different Nanostructures. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–43. [Google Scholar] [CrossRef]
- Darroudi, M.; Yazdi, M.E.T.; Amiri, M.S. Plant-Mediated Biosynthesis of Nanoparticles. In 21st Century Nanoscience—A Handbook; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Amiri, M.S.; Nourbakhsh, F.; Rahnama, M.; Forouzanfar, F.; Mousavi, S.H. Bio-indicators in cadmium toxicity: Role of HSP27 and HSP70. Environ. Sci. Pollut. Res. 2021, 28, 26359–26379. [Google Scholar] [CrossRef] [PubMed]
- Es-haghi, A.; Javadi, F.; Yazdi, M.E.T.; Amiri, M.S. The Expression of Antioxidant Genes and Cytotoxicity of Biosynthesized Cerium Oxide Nanoparticles Against Hepatic Carcinoma Cell Line. Avicenna J. Med. Biochem. 2019, 7, 16–20. [Google Scholar] [CrossRef]
- Amiri, M.S.; Taghavizadeh Yazdi, M.E.; Rahnama, M. Medicinal plants and phytotherapy in Iran: Glorious his-tory, current status and future prospects. Plant Sci. Today 2021, 8, 95–111. [Google Scholar] [CrossRef]
- Alabyadh, T.; Albadri, R.; Es-Haghi, A.; Yazdi, M.E.T.; Ajalli, N.; Rahdar, A.; Thakur, V.K. ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells. J. Funct. Biomater. 2022, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, M.; Abad, M.H.K.; Gholami, A.; Yazdi, M.E.T.; Iriti, M.; Mottaghipisheh, J. Phenolic content and antioxidant activity of different Iranian populations of Anabasis aphylla L. Nat. Prod. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ghorani-Azam, A.; Mottaghipisheh, J.; Amiri, M.S.; Mashreghi, M.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Nourbakhsh, F.; Nadaf, M.; Qayoomian, M.; Yazdi, M.E.T. Resveratrol-Mediated Gold-Nanoceria Synthesis as Green Nanomedicine for Phytotherapy of Hepatocellular Carcinoma. Front. Biosci. 2022, 27, 227. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.J.; Es-Haghi, A.; Yazdi, M.E.T.; Rahdar, A.; Baino, F. MOF-Mediated Synthesis of CuO/CeO2 Composite Nanoparticles: Characterization and Estimation of the Cellular Toxicity against Breast Cancer Cell Line (MCF-7). J. Funct. Biomater. 2021, 12, 53. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Es-Haghi, A.; Yazdi, M.T.; Sharifalhoseini, M.; Baghani, M.; Yousefi, E.; Rahdar, A.; Baino, F. Application of Response Surface Methodology for Optimizing the Therapeutic Activity of ZnO Nanoparticles Biosynthesized from Aspergillus niger. Biomimetics 2021, 6, 34. [Google Scholar] [CrossRef]
- Hashemzadeh, M.R.; Yazdi, M.E.T.; Amiri, M.S.; Mousavi, S.H. Stem cell therapy in the heart: Biomaterials as a key route. Tissue Cell 2021, 71, 101504. [Google Scholar] [CrossRef]
- Mousavi-Kouhi, S.M.; Beyk-Khormizi, A.; Mohammadzadeh, V.; Ashna, M.; Es-haghi, A.; Mashreghi, M.; Hashemzadeh, V.; Mozafarri, H.; Nadaf, M.; Taghavizadeh Yazdi, M.E. Biological synthesis and characterization of gold nanoparticles using Verbascum speciosum Schrad. and cytotoxicity properties toward HepG2 cancer cell line. Res. Chem. Intermed. 2021, 48, 167–178. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Khara, J.; Housaindokht, M.R.; Sadeghnia, H.R.; Bahabadid, S.E.; Amiri, M.S.; Darroudi, M. Biocomponents and Antioxidant Activity of Ribes khorasanicum. Int. J. Basic Sci. Med. 2018, 3, 99–103. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Khara, J.; Housaindokht, M.R.; Sadeghnia, H.R.; Esmaeilzadeh Bahabadi, S.; Amiri, M.S.; Darroudi, M. Assessment of phytochemical components and antioxidant activity of Rheum turkestanicum Janisch. Stud. Med. Sci. 2020, 31, 75–81. [Google Scholar]
- Yazdi, M.E.T.; Amiri, M.S.; Hosseini, H.A.; Oskuee, R.K.; Mosawee, H.; Pakravanan, K.; Darroudi, M. Plant-based synthesis of silver nanoparticles in Handelia trichophylla and their biological activi-ties. Bull. Mater. Sci. 2019, 42, 155. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, M.E.T.; Darroudi, M.; Amiri, M.S.; Hosseini, H.A.; Nourbakhsh, F.; Mashreghi, M.; Farjadi, M.; Kouhi, S.M.M.; Mousavi, S.H. Anticancer, antimicrobial, and dye degradation activity of biosynthesised silver nanoparticle using Artemisia kopetdaghensis. Micro Nano Lett. 2020, 15, 1046–1050. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From plants to antimicrobials: Natural products against bacterial membranes. Phytother. Res. 2021, 36, 33–52. [Google Scholar] [CrossRef]
- Modarres, M.; Yazdi, M.E.T. Elicitation Improves Phenolic Acid Content and Antioxidant Enzymes Activity in Salvia leriifolia Cell Cultures. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 849–855. [Google Scholar] [CrossRef]
- Ashna, M.; Es-Haghi, A.; Noghondar, M.K.; Al Amara, D.; Yazdi, M.E.T. Greener synthesis of cerium oxide nanoemulsion using pollen grains of Brassica napus and evaluation of its antitumour and cytotoxicity properties. Mater. Technol. 2020, 37, 525–532. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Darroudi, M.; Amiri, M.S.; Zarrinfar, H.; Hosseini, H.A.; Mashreghi, M.; Mozafarri, H.; Ghorbani, A.; Mousavi, S.H. Antimycobacterial, Anticancer, Antioxidant and Photocatalytic Activity of Bio-synthesized Silver Nanoparticles Using Berberis Integerrima. Iran. J. Sci. Technol. Trans. A Sci. 2021, 46, 1–11. [Google Scholar]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Nourbakhsh, F.; Mashreghi, M.; Mousavi, S.H. Ultrasound-based synthesis of ZnO·Ag2O3 nanocomposite: Characterization and evaluation of its antimicrobial and anticancer properties. Res. Chem. Intermed. 2021, 47, 1285–1296. [Google Scholar] [CrossRef]
- Mousavi-Kouhi, S.M.; Beyk-Khormizi, A.; Amiri, M.S.; Mashreghi, M.; Yazdi, M.E.T. Silver-zinc oxide nanocomposite: From synthesis to antimicrobial and anticancer properties. Ceram. Int. 2021, 47, 21490–21497. [Google Scholar] [CrossRef]
- Pomeroy, J.E.; Helfer, A.; Bursac, N. Biomaterializing the promise of cardiac tissue engineering. Biotechnol. Adv. 2019, 42, 107353. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, J.; Liao, Y.; Alakpa, E.V.; Bunpetch, V.; Zhang, J.; Ouyang, H. Current advances in microsphere based cell culture and tissue engineering. Biotechnol. Adv. 2019, 39, 107459. [Google Scholar] [CrossRef]
- Hashemzadeh, M.R.; Mahdavi-Shahri, N.; Bahrami, A.R.; Kheirabadi, M.; Naseri, F.; Atighi, M. Use of an in vitro model in tissue engineering to study wound repair and differentiation of blastema tissue from rabbit pinna. Vitr. Cell. Dev. Biol. - Anim. 2015, 51, 680–689. [Google Scholar] [CrossRef]
- Sun, J.; Xu, W.; Zheng, S.; Lv, C.; Lin, J.; Chen, S.; Qiu, Y.; Jiang, X.; Draz, E.; Wang, S. Icariin promotes mouse Leydig cell testosterone synthesis via the Esr1/Src/Akt/Creb/Sf-1 pathway. Toxicol. Appl. Pharmacol. 2022, 441, 115969. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, S.; Wang, J.; Zhang, X.; Yuan, D.; Zhang, C.; Liu, C.; Wang, T.; Zhou, Z. Icariin improves brain function decline in aging rats by enhancing neuronal autophagy through the AMPK/mTOR/ULK1 pathway. Pharm. Biol. 2021, 59, 181–189. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Mei, Q.; Lu, T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015, 126, 57–68. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Xiong, J.; Zhao, J.; Guo, M.; Chen, J.; Zhao, X.; Chen, C.; He, Z.; Zhou, Y.; et al. Icariin as an emerging candidate drug for anticancer treatment: Current status and perspective. Biomed. Pharmacother. 2023, 157. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, X.; Liu, M.; Wang, Y.; Li, D.; Li, X.; Xiao, Y.; Wang, Y.; Zhang, X. The controlled release of a novel thiolated icariin for enhanced osteoporotic bone regeneration. Mater. Des. 2021, 200, 109468. [Google Scholar] [CrossRef]
- Du, W.; Tang, Z.; Yang, F.; Liu, X.; Dong, J. Icariin attenuates bleomycin-induced pulmonary fibrosis by targeting Hippo/YAP pathway. Biomed. Pharmacother. 2021, 143, 112152. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, M.; Wang, L.; Zhou, L.; Feng, X.; Ye, C.; Wang, C. Icariin attenuates renal fibrosis in chronic kidney disease by inhibiting interleukin-1β/transforming growth factor-β-mediated activation of renal fibroblasts. Phytother. Res. 2021, 35, 6204–6215. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and Its Metabolites as Potential Protective Phytochemicals Against Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 00271. [Google Scholar] [CrossRef]
- Pharmacopoeia, C. Chinese Pharmacopoeia Commission Chinese Pharmacopoeia; Chinese Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- The Plant List, 2013. Version 1.1. Published on the Internet. Available online: http://www.theplantlist.org/ (accessed on 1 January 2023).
- Shen, P.; Guo, B.; Gong, Y.; Hong, D.Y.; Hong, Y.; Yong, E. Taxonomic, genetic, chemical and estrogenic characteristics of Epimedium species. Phytochemistry 2007, 68, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Yan-Jun, Z.; Jian-Qiang, L. A New Species of Epimedium (Berberidaceae) from Hubei, China. Novon 2009, 19, 567–569. [Google Scholar] [CrossRef]
- Liu, C.; Xu, L. Analysis of active ingredients of traditional Chinese herbal drug. Assay of icariin in Epimedium. Chin J Pharm Anal 1984, 4, 81–84. [Google Scholar]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Zhai, Y.-K.; Guo, X.; Pan, Y.-L.; Niu, Y.-B.; Li, C.-R.; Wu, X.-L.; Mel, Q.-B. A systematic review of the efficacy and pharmacological profile of Herba Epimedii in osteoporosis therapy. Die Pharm. 2013, 68, 713–722. [Google Scholar]
- Aljehani, A.A.; Albadr, N.A.; Nasrullah, M.Z.; Neamatallah, T.; Eid, B.G.; Abdel-Naim, A.B. Icariin ameliorates metabolic syndrome-induced benign prostatic hyperplasia in rats. Environ. Sci. Pollut. Res. 2021, 29, 20370–20378. [Google Scholar] [CrossRef]
- Hou, L.; Lin, Z.; Xu, A.; Le, G.; Ge, L.; Liu, S.; Muhmood, A.; Gan, F.; Huang, K. Combined protective effects of icariin and selenomethionine on novel chronic tubulointerstitial nephropathy models in vivo and in vitro. Br. J. Nutr. 2021, 127, 12–22. [Google Scholar] [CrossRef]
- Kou, Z.; Wang, C.; Gao, L.; Chu, G.; Yang, G.; Pang, W. Icariin improves pig sperm quality through antioxidant and antibacterial effects during liquid storage at 17 °C. Livest. Sci. 2022, 256, 104827. [Google Scholar] [CrossRef]
- Yuan, J.-Y.; Yuan, J.-Y.; Tong, Z.-Y.; Dong, Y.-C.; Zhao, J.-Y.; Shang, Y. Research progress on icariin, a traditional Chinese medicine extract, in the treatment of asthma. Allergologia Immunopathol. 2022, 50, 9–16. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Xiao, W.; Ji, Q.; Liu, Y. Effects of icariin on NRG1-ErbB signaling pathways in hippocampus of schizophrenia rats. Chin. J. Tissue Eng. Res. 2023, 27, 3236–3241. [Google Scholar]
- Lu, C.; Zou, K.; Guo, B.; Li, Q.; Wang, Z.; Xiao, W.; Zhao, L. Linker-peptide-mediated one-step purification and immobilization of alpha-L-rhamnosidase from Bacteroides thetaiotaomicron for direct biotransformation from epimedin C to icariin. Enzym. Microb. Technol. 2023, 162, 110131. [Google Scholar] [CrossRef]
- Mei, J.; He, Q.; Sun, X.; Yin, H.; Qian, W.Q. Icariin promotes osteoblast proliferation and differentiation through a non-nuclear signaling pathway. Chinese J. Tissue Eng. Res. 2023, 27, 3129–3135. [Google Scholar]
- Xue, P.; Du, B.; Liu, X.; Sun, G.; Cheng, T.; Chen, H.; He, S. Characterization and osteogenic ability of Mg-F membrane/icariin membrane/beta-tricalcium phosphate scaffolds fabricated by coating process combined with 3D printing. Chin. J. Tissue Eng. Res. 2023, 27, 2480–2487. [Google Scholar]
- Yan, F.; Liu, J.; Chen, M.-X.; Zhang, Y.; Wei, S.-J.; Jin, H.; Nie, J.; Fu, X.-L.; Shi, J.-S.; Zhou, S.-Y.; et al. Icariin ameliorates memory deficits through regulating brain insulin signaling and glucose transporters in 3?Tg-AD mice. Neural Regen. Res. 2023, 18, 183–188. [Google Scholar]
- Chen, M.; Wu, J.; Luo, Q.; Mo, S.; Lyu, Y.; Wei, Y.; Dong, J. The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II. Nutrients 2016, 8, 563. [Google Scholar] [CrossRef] [Green Version]
- Iqubal, M.K.; Chaudhuri, A.; Iqubal, A.; Saleem, S.; Gupta, M.M.; Ahuja, A.; Ali, J.; Baboota, S. Targeted Delivery of Natural Bioactives and Lipid-nanocargos against Signaling Pathways Involved in Skin Cancer. Curr. Med. Chem. 2021, 28, 8003–8035. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; Mehta, D.K. Phyto-Phospholipid Complexation as a Novel Drug Delivery System for Management of Cancer with Better Bioavailability: Current Perspectives and Future Prospects. Anti-Cancer Agents Med. Chem. 2021, 21, 1403–1412. [Google Scholar] [CrossRef]
- He, Q.; Dong, H.; Gong, M.; Guo, Y.; Xia, Q.; Gong, J.; Lu, F. New Therapeutic Horizon of Graves’ Hyperthyroidism: Treatment Regimens Based on Immunology and Ingredients from Traditional Chinese Medicine. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Liu, J.-P. Novel strategies for molecular targeting to cancer. Clin. Experim. Pharmacol. Physiol. 2016, 43, 287–289. [Google Scholar] [CrossRef]
- Abbas, M.N.; Kausar, S.; Cui, H. Therapeutic potential of natural products in glioblastoma treatment: Targeting key glioblas-toma signaling pathways and epigenetic alterations. Clin. Transl. Oncol. 2020, 22, 963–977. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Alharbi, W.S.; Alfaleh, M.A.; Al-Hejaili, O.D.; Aldawsari, H.M.; Eid, B.G.; Bakhaidar, R.; Drago, F.; Caraci, F.; et al. Development of an Icariin-Loaded Bilosome-Melittin Formulation with Improved Anticancer Activity against Cancerous Pancreatic Cells. Pharmaceuticals 2021, 14, 1309. [Google Scholar] [CrossRef]
- Agarwal, S.; Sau, S.; Iyer, A.K.; Dixit, A.; Kashaw, S.K. Multiple strategies for the treatment of invasive breast carcinoma: A comprehensive prospective. Drug Discov. Today 2022, 27, 585–611. [Google Scholar] [CrossRef]
- Almoshari, Y. Development, Therapeutic Evaluation and Theranostic Applications of Cubosomes on Cancers: An Up-dated Review. Pharmaceutics 2022, 14, 600. [Google Scholar] [CrossRef]
- Wu, J.-F.; Dong, J.-C.; Xu, C.-Q. Effects of icariin on inflammation model stimulated by lipopolysaccharide in vitro and in vivo. Chin. J. Integr. Tradit. West. Med. 2009, 29, 330–334. [Google Scholar]
- Yasukawa, K.; Ko, S.-K.; Whang, W.-K. Inhibitory effects of the aerial parts of Epimedium koreanum on TPA-induced inflammation and tumour promotion in two-stage carcinogenesis in mouse skin. J. Pharm. Nutr. Sci. 2016, 6, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Hua, W.; Zhang, Y.; Wu, X.; Kang, L.; Tu, J.; Zhao, K.; Li, S.; Wang, K.; Song, Y.; Luo, R.; et al. Icariin attenuates interleukin-1beta-induced inflammatory response in human nucleus pulposus cells. Curr. Pharm. Des. 2017, 23, 6071–6078. [Google Scholar] [CrossRef]
- Zhong, S.; Ge, J.; Yu, J.-Y. Icariin prevents cytokine-induced beta-cell death by inhibiting NF-kappaB signaling. Exp. Ther. Med. 2018, 16, 2752–2762. [Google Scholar]
- Li, X.; Zhu, T.; Wang, M.; Zhang, F.; Zhang, G.; Zhao, J.; Zhang, Y.; Wu, E.; Li, X. Icariin Attenuates M1 Activation of Microglia and Abeta Plaque Accumulation in the Hippocampus and Prefrontal Cortex by Up-Regulating PPARgamma in Restraint/Isolation-Stressed APP/PS1 Mice. Front. Neurosci. 2019, 13, 291. [Google Scholar]
- Ma, A.; Ma, A.; You, Y.; Chen, B.; Wang, W.; Liu, J.; Qi, H.; Liang, Y.; Li, Y.; Li, C. Icariin/aspirin composite coating on TiO2 nanotubes surface induce immunomodulatory effect of macro-phage and improve osteoblast activity. Coatings 2020, 10, 427. [Google Scholar] [CrossRef]
- Desai, T.D.; Wen, Y.-T.; Daddam, J.R.; Cheng, F.; Chen, C.-C.; Pan, C.-L.; Lin, K.-L.; Tsai, R.-K. Long term therapeutic effects of icariin-loaded PLGA microspheres in an experimental model of optic nerve ischemia via modulation of CEBP-beta/G-CSF/noncanonical NF-kappaB axis. Bioeng. Transl. Med 2022, 7, e10289. [Google Scholar] [CrossRef]
- Liu, T.; Xin, H.; Li, W.; Zhou, F.; Li, G.; Gong, Y.; Gao, Z.; Qin, X.; Cui, W.; Shindel, A.W.; et al. Effects of Icariin on Improving Erectile Function in Streptozotocin-Induced Diabetic Rats. J. Sex. Med. 2011, 8, 2761–2772. [Google Scholar] [CrossRef]
- Niu, Y.; Lin, G.; Pan, J.; Liu, J.; Xu, Y.; Cai, Q.; Wang, T.; Luan, Y.; Chen, Y.; Feng, Y.; et al. Deciphering the myth of icariin and synthetic derivatives in improving erectile function from a molecular biology perspective: A narrative review. Transl. Androl. Urol. 2021. [Google Scholar] [CrossRef]
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of Icariin on Reproductive Functions in Male Rats. Molecules 2014, 19, 9502–9514. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xin, H.; Wu, Y.; Guan, R.; Lei, H.; Fu, X.; Xin, Z.; Yang, Y. Effect of icariin in combination with daily sildenafil on penile atrophy and erectile dysfunction in a rat model of bilateral cavernous nerves injury. Andrology 2017, 5, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Yu, C.; Lu, Q.; Li, H.; Li, Z.; He, C. Mechanism of Action of Icariin in Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Q.; Wu, X.; Zhu, H.; Deng, X.; Wang, M.; Yang, S.; Xu, J.; Chen, Q.; Li, M. Icariin Treatment Rescues Diabetes Induced Bone Loss via Scavenging ROS and Activating Primary Cilia/Gli2/Osteocalcin Signaling Pathway. Cells 2022, 11, 4091. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Qu, R.; Yang, Y.; Gong, X.; Hong, Y.; Jin, A.; Huang, X.; Dai, Q.; Jiang, L. Icariin prevents oestrogen deficiency–induced alveolar bone loss through promoting osteogenesis via STAT3. Cell Prolif. 2020, 53, e12743. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; He, M.; Chen, M.; Chen, Z.; Yang, F.; Wang, H.; Zhang, J.; He, W. Icariin stimulates osteogenic differentiation of rat bone marrow stromal stem cells by increasing TAZ expression. Biomed. Pharmacother. 2017, 91, 581–589. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, F.; Feng, Y.; Zhang, S.; Xie, C.; Huang, H.; Sang, C.; Hu, S.; Jiao, F.; Jiang, J. Icariin regulates stem cell migration for endogenous repair of intervertebral disc degeneration by in-creasing the expression of chemotactic cytokines. BMC Complem. Med. Ther. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Bian, Q.; Liu, S.; Zhao, Y.; Huang, J.; Shen, Z. Icariin promotes osteoblastic differentiation in OVX mice via MAPK signaling pathway revealed by profiling. Tradit. Med. Mod. Med. 2018, 1, 33–41. [Google Scholar] [CrossRef]
- Xin, G.; Yuedong, Y.; Xuemei, S.; Chenhan, M.; Meng, Z.; Chenbo, Z.; Ning, G.; Xindong, W. The mechanism of Epimedium in the treatment of coronary atherosclerotic heart disease based on network pharmacology, molecular docking, and in vitro studies. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2478–2488. [Google Scholar]
- Sharma, S.; Khan, V.; Dhyani, N.; Najmi, A.; Haque, S. Icariin attenuates isoproterenol-induced cardiac toxicity in Wistar rats via modulating cGMP level and NF-κB signaling cascade. Human Exper. Toxicol. 2020, 39, 117–126. [Google Scholar] [CrossRef]
- Zhai, M.; Minghe, Z.; Ju, X.; Shao, L.; Ling, S.; Zhang, Y.; Liu, Y.; Zhao, H. Icariin Acts as a Potential Agent for Preventing Cardiac Ischemia/Reperfusion Injury. Cell Biochem. Biophys. 2015, 72, 589–597. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiong, Y.; Yang, T.; Wang, Y.; Zeng, J.; Zhou, S.; Luo, Y.; Li, L. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: From effects to molecular mechanisms. Biomed. Pharmacother. 2022, 147, 112642. [Google Scholar] [CrossRef]
- Ni, T.; Lin, N.; Huang, X.; Lu, W.; Sun, Z.; Zhang, J.; Lin, H.; Chi, J.; Guo, H. Icariin Ameliorates Diabetic Cardiomyopathy Through Apelin/Sirt3 Signalling to Improve Mitochondrial Dysfunction. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, W.; Lin, Q.; Wang, W. Icariin influences cardiac remodeling following myocardial infarction by regulating the CD147/MMP-9 pathway. J. Int. Med. Res. 2018, 46, 2371–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Z.; Liu, J.; Xu, P.; Gao, A.; Wang, L.; Ji, L. The Cardioprotective Effect of Icariin on Ischemia–Reperfusion Injury in Isolated Rat Heart: Potential Involvement of the PI 3 K-A kt Signaling Pathway. Cardiovasc. Ther. 2015, 33, 134–140. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Galli, G.V.; Cero, E.D.; Belluti, F.; Matera, R.; Zironi, E.; Pagliuca, G.; Bosisio, E. Potent Inhibition of Human Phosphodiesterase-5 by Icariin Derivatives. J. Nat. Prod. 2008, 71, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.H.; Bin Jia, X.; Hu, M. Intestinal Absorption Mechanisms of Prenylated Flavonoids Present in the Heat-Processed Epimedium koreanum Nakai (Yin Yanghuo). Pharm. Res. 2008, 25, 2190–2199. [Google Scholar] [CrossRef]
- Ding, L.; Liang, X.; Zhu, D.; Lou, Y. Icariin promotes expression of PGC-1α, PPARα, and NRF-1 during cardiomyocyte differentiation of murine embryonic stem cells in vitro 1. Acta Pharm. Sin. 2007, 28, 1541–1549. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.X.; Chen, Z.Q.; Liu, Z.J.; Dang, G.T. Icariine stimulates proliferation and differentiation of human osteoblasts by increasing production of bone morphogenetic protein 2. Chin. Med. J. 2007, 120, 204–210. [Google Scholar] [CrossRef]
- Hsiung, S.C.; Adlersberg, M.; Arango, V.; Mann, J.J.; Tamir, H.; Liu, K.P. Attenuated 5-HT1A receptor signaling in brains of suicide victims: Involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. J. Neurochem. 2003, 87, 182–194. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Wang, M.; Gao, H.; Li, W.; Wu, B. Icariin and its metabolites regulate lipid metabolism: From effects to molecular mechanisms. Biomed. Pharmacother. 2020, 131, 110675. [Google Scholar] [CrossRef]
- Zhang, X.D.; Guo, Y.; Li, D.X.; Wang, R.; Fan, H.S.; Xiao, Y.M.; Zhang, L.; Zhang, X.D. The effect of loading icariin on biocompatibility and bioactivity of porous β-TCP ceramic. J. Mater. Sci. Mater. Med. 2011, 22, 371–379. [Google Scholar] [CrossRef]
- Choi, S.; Noh, S.; Lim, C.; Kim, H.-J.; Jo, H.-S.; Min, J.; Park, K.; Kim, S. Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro. Nanomaterials 2020, 10, 2071. [Google Scholar] [CrossRef]
- Oprita, E.I.; Iosageanu, A.; Craciunescu, O. Progress in Composite Hydrogels and Scaffolds Enriched with Icariin for Osteochondral Defect Healing. Gels 2022, 8, 648. [Google Scholar] [CrossRef]
- Cui, Y.-L.; Zhang, Y.; Meng, F.-C.; Lin, K.-M.; Wang, Q.-S. Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int. J. Nanomed. 2012, 7, 4239–4249. [Google Scholar] [CrossRef] [Green Version]
- Otto, W.; Rao, J. Tomorrow’s skeleton staff: Mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004, 37, 97–110. [Google Scholar] [CrossRef]
- Li, W.-J.; Tuli, R.; Huang, X.; Laquerriere, P.; Tuan, R.S. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 2005, 26, 5158–5166. [Google Scholar] [CrossRef]
- Cowan, C.M.; Shi, Y.-Y.; Aalami, O.; Chou, Y.-F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef]
- Datta, N.; Pham, Q.P.; Sharma, U.; Sikavitsas, V.I.; Jansen, J.A.; Mikos, A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 2488–2493. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Vehof, J.; van den Dolder, J.; de Ruijter, J.E.; Spauwen, P.H.M.; Jansen, J.A. Bone formation in CaP-coated and noncoated titanium fiber mesh. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. 2003, 64, 417–426. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Yang, S.; Rutherford, R.B.; Krebsbach, P.H.; Zhao, M.; Wang, D. Gene Therapy Approaches for Bone Regeneration. Cells Tissues Organs 2004, 176, 95–108. [Google Scholar] [CrossRef]
- Byers, B.A.; Guldberg, R.E.; García, A.J. Synergy between Genetic and Tissue Engineering: Runx2 Overexpression and in vitro Construct Development Enhance In Vivo Mineralization. Tissue Eng. 2004, 10, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T. The role of growth factors in the repair of bone: Biology and clinical applications. JBJS 2002, 84, 1032–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridwell, K.; Anderson, P.; Boden, S.; Vaccaro, A.; Wang, J. What’s New in Spine Surgery. Spine Surg. 2009, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.-I.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin Potentiates the in Vivo Ectopic Bone Formation Induced by Bone Morphogenetic Protein-2. J. Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of Bone Formation in Vitro and in Rodents by Statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R. In Vitro and In Vivo effects of ipriflavone on bone formation and bone biomechanics. Calcif. Tissue Int. 1997, 61, S12–S14. [Google Scholar] [CrossRef]
- Zou, L.; Hu, L.; Pan, P.; Tarafder, S.; Du, M.; Geng, Y.; Xu, G.; Chen, L.; Chen, J.; Lee, C.H. Icariin-releasing 3D printed scaffold for bone regeneration. Compos. Part B Eng. 2022, 232, 109625. [Google Scholar] [CrossRef]
- Zhou, L.; Poon, C.C.-W.; Wong, K.-Y.; Cao, S.; Dong, X.; Zhang, Y.; Wong, M.-S. Icariin ameliorates estrogen-deficiency induced bone loss by enhancing IGF-I signaling via its crosstalk with non-genomic ERα signaling. Phytomedicine 2021, 82, 153413. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Y.; Jia, B.; Wang, Y.; Li, T. Icariin stimulates osteogenesis and suppresses adipogenesis of human bone mesenchymal stem cells via miR-23a-mediated activation of the Wnt/β-catenin signaling pathway. Phytomedicine 2021, 85, 153485. [Google Scholar] [CrossRef]
- Dong, M.; Wu, S.; Xu, H.; Yu, X.; Wang, L.; Bai, H.; Niu, W. FBS-Derived Exosomes as a Natural Nano-Scale Carrier for Icariin Promote Osteoblast Proliferation. Front. Bioeng. Biotechnol. 2021, 9, 615920. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Li, H.; Jia, L.; Wang, J.; Liang, S.; Cai, A.; Tan, X.; Wang, L.; Wang, X.; et al. Verification of pain-related neuromodulation mechanisms of icariin in knee osteoarthritis. Biomed. Pharmacother. 2021, 144, 112259. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2014, 11, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of Agarose/Silk Fibroin Blended Hydrogel for in Vitro Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Madry, H.; Cucchiarini, M. Hydrogel-based controlled delivery systems for articular cartilage repair. BioMed Res. Int. 2016, 2016, 1215263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Liu, Y.; He, L.; Wang, Q.; Wang, L.; Yuan, T.; Xiao, Y.; Fan, Y.; Zhang, X. Icariin conjugated hyaluronic acid/collagen hydrogel for osteochondral interface restoration. Acta Biomater. 2018, 74, 156–167. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Luo, Z.; Li, D.; Lu, J.; Wang, Q.; Xiao, Y.; Zhang, X. Development of an injectable thiolated icariin functionalized collagen/hyaluronic hydrogel to promote cartilage formation in vitro and in vivo. J. Mater. Chem. B 2019, 7, 2845–2854. [Google Scholar] [CrossRef]
- Yuan, T.; He, L.; Yang, J.; Zhang, L.; Xiao, Y.; Fan, Y.; Zhang, X. Conjugated icariin promotes tissue-engineered cartilage formation in hyaluronic acid/collagen hydrogel. Process. Biochem. 2015, 50, 2242–2250. [Google Scholar] [CrossRef]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regen-eration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Kankala, R.K.; Lu, F.-J.; Liu, C.-G.; Zhang, S.-S.; Chen, A.-Z.; Wang, S.-B. Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair. Materials 2018, 11, 1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.-J.; Cao, L.-G.; Wu, T.; Wang, D.-X.; Jin, D.; Jiang, S.; Zhang, Z.-Y.; Bi, L.; Pei, G.-X. The Dose-Effect of Icariin on the Proliferation and Osteogenic Differentiation of Human Bone Mesenchymal Stem Cells. Molecules 2011, 16, 10123–10133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Yang, J.; Lu, J.; Xiao, Y.; Fan, Y.; Zhang, X. Preparation and characterization of a novel hyaluronic acid–icariin conjugate hydrogel. Mater. Lett. 2014, 136, 41–44. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, L.; Xia, L.; Wu, Q.; Wang, J.; Wang, X.; Xu, L.; Zhou, Y.; Xu, Y.; Jiang, X. Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, K.; Sun, H.; Wang, J.; Fu, Z.; Liu, M. Icariin promotes stable chondrogenic differentiation of bone marrow mesenchymal stem cells in self-assembling peptide nanofiber hydrogel scaffolds. Mol. Med. Rep. 2018, 17, 8237–8243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ye, L.; Cai, X.; Li, Z.; Fan, Y.; Yang, F. Icariin-Loaded Hydrogel Regulates Bone Marrow Mesenchymal Stem Cell Chondrogenic Differentiation and Promotes Cartilage Repair in Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- Green, D.R. Means to an End: Apoptosis and Other Cell Death Mechanisms; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2011. [Google Scholar]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Lee, L.-H.; Goh, B.-H. Anti-Cancer Properties of the Naturally Occurring Aphrodisiacs: Icariin and Its Derivatives. Front. Pharmacol. 2016, 7, 191. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Hao, J.; Pu, J.; Zhao, L.; Lü, Z.; Hu, J.; Yu, Q.; Wang, Y.; Xie, Y.; Li, G. Icariin induces apoptosis in mouse MLTC-10 Leydig tumor cells through activation of the mitochon-drial pathway and down-regulation of the expression of piwil4. Int. J. Oncol. 2011, 39, 973–980. [Google Scholar]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E.; et al. Human Cartilage Repair with a Photoreactive Adhesive-Hydrogel Composite. Sci. Transl. Med. 2013, 5, 167ra6. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Yang, Y.; Liu, Y.; Jiang, S.; Di, S.; Hu, W.; Ma, Z.; Li, T.; Zhu, Y.; Xin, Z.; et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci. Rep. 2016, 6, 21145. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020, 111, 4242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, J.; Li, Q.; Ming, W.; Fu, Y.; Song, L.; Qin, J. Study on the regulatory mechanism and experimental verification of icariin for the treatment of ovarian cancer based on network pharmacology. J. Ethnopharmacol. 2020, 262, 113189. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Xiang, G. Engineering nanomedicines to inhibit hypoxia-inducible Factor-1 for cancer therapy. Cancer Lett. 2022, 530, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, S.; Jiang, L.; Zhang, S.; Li, W.; Chen, Z.; Zhang, D. Expression of RECK and MMP-2 in salivary adenoid cystic carcinoma: Correlation with tumor progression and patient prognosis. Oncol. Lett. 2014, 7, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Zhang, L.; Wang, Y.; Li, X. Experimental studies of icariin on anticancer mechanism. J. Chin. Med. Mater. 2000, 23, 554–556. [Google Scholar]
- Wang, Y.; Dong, H.; Zhu, M.; Ou, Y.; Zhang, J.; Luo, H.; Luo, R.; Wu, J.; Mao, M.; Liu, X. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur. J. Pharmacol. 2010, 635, 40–48. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Zhang, T.; Ye, X.; Sun, X.; Zhang, L.-L.; Wu, C.-J. Natural substances derived from herbs or plants are promising sources of anticancer agents against colorectal cancer via triggering apoptosis. J. Pharm. Pharmacol. 2021, 74, 162–178. [Google Scholar] [CrossRef]
- Zou, J.; Xu, M.; Li, F.; Wang, Y.; Li, X.; Yu, D.; Ma, Y.; Zhang, Y.; Sun, X. Icaritin alleviates docetaxel-induced skin injury by suppressing reactive oxygen species via estrogen receptors. Thorac. Cancer 2021, 13, 190–201. [Google Scholar] [CrossRef]
- Deng, J.; Wang, J.; Shi, J.; Li, H.; Lu, M.; Fan, Z.; Gu, Z.; Cheng, H. Tailoring the physicochemical properties of nanomaterials for immunomodulation. Adv. Drug Deliv. Rev. 2021, 180, 114039. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Miao, Y.; Wang, L.; Yin, H. Sex hormone-like Effects of Icariin on T-cells immune modulation in spontaneously hypertensive rats. J. Ethnopharmacol. 2020, 269, 113717. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.T.; Carmeli, Y.; Falagas, M.T.; Giske, C.T.; Harbarth, S.; Hindler, J.T.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Chen, W.; Qu, L.; Wu, J.; Si, J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and P-glycoprotein expression. Mol. Med. Rep. 2013, 8, 1883–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Wang, Y.; Ribeiro, C.F.; Manokaran, C.; Chang, H.; Von, T.; Rodrigues, S.; Cizmecioglu, O.; Jia, S.; Korpal, M.; et al. Blocking PI3K p110β Attenuates Development of PTEN-Deficient Castration-Resistant Prostate Cancer. Mol. Cancer Res. 2022, 20, 673–685. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Nukolova, N.; Laquer, F.C.; Poluektova, L.; Alnouti, Y.; Yokohira, M.; Arnold, L.L.; Kabanov, A.; Cohen, S.M.; Bronich, T.K.; et al. Cisplatin-loaded core cross-linked micelles: Comparative pharmacokinetics, antitumor activity, and toxicity in mice. Int. J. Nanomed. 2012, 7, 2557–2571. [Google Scholar] [CrossRef] [Green Version]
- Launay-Vacher, V.; Rey, J.-B.; Isnard-Bagnis, C.; Deray, G.; Daouphars, M. Prevention of cisplatin nephrotoxicity: State of the art and recommendations from the Eu-ropean Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother. Pharmacol. 2008, 61, 903–909. [Google Scholar] [CrossRef]
- Ma, Z.-n.; Liu, Z.; Wang, Z.; Ren, S.; Tang, S.; Wang, Y.-p.; Xiao, S.-y.; Chen, C.; Li, W. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-κB signaling pathways. Food Chem. Toxicol. 2017, 110, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Arivarasu, N.; Priyamvada, S.; Mahmood, R. Oral administration of caffeic acid ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Exp. Toxicol. Pathol. 2013, 65, 21–25. [Google Scholar] [CrossRef]
- Van Angelen, A.A.; Glaudemans, B.; van der Kemp, A.W.; Hoenderop, J.G.; Bindels, R.J. Cisplatin-induced injury of the renal distal convoluted tubule is associated with hypomagnesaemia in mice. Nephrol. Dial. Transplant. 2012, 28, 879–889. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Zhang, S.; Su, X.; Qiu, G.; Wu, Z. Protective effects of icariin on cisplatin-induced acute renal injury in mice. Am. J. Transl. Res. 2015, 7, 2105–2114. [Google Scholar] [PubMed]
- Jiang, S.; Chang, H.; Deng, S.; Fan, D. Icariin enhances the chemosensitivity of cisplatin-resistant ovarian cancer cells by suppressing au-tophagy via activation of the AKT/mTOR/ATG5 pathway. Int. J. Oncol. 2019, 54, 1933–1942. [Google Scholar] [PubMed]
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr. Protoc. Pharmacol. 2019, 86, e67. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Xin, Z.-C.; Xin, H.; Yuan, Y.-M.; Tian, L.; Guo, Y.-L. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J. Androl. 2005, 7, 381–388. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y. Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-Y.; Jiang, J.-G.; Yang, L.; Wang, D.-W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2016, 174, 1395–1425. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sun, T.; Wu, J.; Kalionis, B.; Zhang, C.; Yuan, D.; Huang, J.; Cai, W.; Fang, H.; Xia, S. Icariin intervenes in cardiac inflammaging through upregulation of SIRT6 enzyme activity and inhibition of the NF-kappa B pathway. BioMed Res. Int. 2015, 2015, 895976. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; He, H. Effects of icariin on expression of glucose regulated protein 78 in vascular smooth muscle cell in-duced by homocysteine. China J. Chin. Mater. Med. 2009, 34, 1964–1967. [Google Scholar]
- Yang, H.; Yan, L.; Qian, P.; Duan, H.; Wu, J.; Li, B.; Wang, S. Icariin Inhibits Foam Cell Formation by Down-Regulating the Expression of CD36 and Up-Regulating the Expression of SR-BI. J. Cell. Biochem. 2014, 116, 580–588. [Google Scholar] [CrossRef]
- Xu, C.-Q.; Liu, B.-J.; Wu, J.-F.; Xu, Y.-C.; Duan, X.-H.; Cao, Y.-X.; Dong, J.-C. Icariin attenuates LPS-induced acute inflammatory responses: Involvement of PI3K/Akt and NF-κB signaling pathway. Eur. J. Pharmacol. 2010, 642, 146–153. [Google Scholar] [CrossRef]
- Calderon-Garcidueñas, A.L.; Duyckaerts, C. Alzheimer disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–337. [Google Scholar]
- Guo, J.; Li, F.; Wu, Q.; Gong, Q.; Lu, Y.; Shi, J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine 2010, 17, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Chen, B.; Wang, X.; Gao, C.; Yu, H. Icariin enhance mild hypothermia-induced neuroprotection via inhibiting the activation of NF-κB in experimental ischemic stroke. Metab. Brain Dis. 2021, 36, 1179–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rong, Y.; Luo, L. Neuroprotective effects of icariin in neonatal hypoxia-ischemic brain damage via its anti-apoptotic property. Child’s Nerv. Syst. 2020, 37, 39–46. [Google Scholar] [CrossRef]
- Zou, X.; Feng, X.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C.; Zhang, Y. Icariin Attenuates Amyloid-β (Aβ)-Induced Neuronal Insulin Resistance Through PTEN Downregulation. Front. Pharmacol. 2020, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Shi, P.; Liu, Y.; Li, T.; Liu, S.; Wang, C.; Wang, L.; Cao, Y. Icariin treatment reduces blood glucose levels in type 2 diabetic rats and protects pancreatic function. Exp. Ther. Med. 2020, 19, 2690–2696. [Google Scholar] [CrossRef] [Green Version]
- Qiao, C.; Wang, H.; Song, Z.; Ding, Y.; Tao, J.; Aa, J.; Ding, X. Icariin Attenuates Diabetic Cardiomyopathy and Downregulates Extracellular Matrix Proteins in Heart Tissue of Type 2 Diabetic Rats. Pharmacology 2020, 105, 576–585. [Google Scholar] [CrossRef]
- Qi, C.; Shao, Y.; Liu, X.; Wang, D.; Li, X. The cardioprotective effects of icariin on the isoprenaline-induced takotsubo-like rat model: Involvement of reactive oxygen species and the TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 74, 105733. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Wang, X.; Sun, J.; Li, Z. Icariin exerts a protective effect against d-galactose induced premature ovarian failure via promoting DNA damage repair. Biomed. Pharmacother. 2019, 118, 109218. [Google Scholar] [CrossRef]
- Ni, G.; Zhang, X.; Afedo, S.Y.; Rui, R. Evaluation of the protective effects of icariin on nicotine-induced reproductive toxicity in male mouse—A pilot study. Reprod. Biol. Endocrinol. 2020, 18, 1–8. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, B.; Zhang, C.; Wang, X.-M.; Zhen, D.; Huang, X.-M.; Chen, W.; Gao, J.-M. Effects of icariin on ovarian function in d-galactose-induced aging mice. Theriogenology 2018, 125, 157–167. [Google Scholar] [CrossRef]
- Song, L.; Liu, C.; Zhu, S.; Chen, H.; Zhang, Q. Icariin Suppresses Proliferation and Metastasis and Enhances Antitumor Immunity in Triple-Negative Breast Cancer via SIRT6/NF-κB Signaling Pathway. Authorea Preprints. 2020. Available online: https://www.authorea.com/users/314916/articles/445270-icariin-suppresses-proliferation-and-metastasis-and-enhances-antitumor-immunity-in-triple-negative-breast-cancer-via-sirt6-nf-%CE%BAb-signaling-pathway (accessed on 1 January 2023).
- Wu, X.; Kong, W.; Qi, X.; Wang, S.; Chen, Y.; Zhao, Z.; Wang, W.; Lin, X.; Lai, J.; Yu, Z.; et al. Icariin induces apoptosis of human lung adenocarcinoma cells by activating the mitochondrial apoptotic pathway. Life Sci. 2019, 239, 116879. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Seo, J.H.; Lee, K.Y.; Park, B. Icariin sensitizes human colon cancer cells to TRAIL-induced apoptosis via ERK-mediated upregulation of death receptors. Int. J. Oncol. 2020, 56, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, P.-Y.; Zhang, Z.-F.; Qin, F.-W.; Tang, W.; Liu, D.-H. Icariin interferes with TDP43-induced inflammatory factor secretion and inhibits the JNK and p38 MAPK signaling pathway in vitro. Arch. Med Sci. 2021. [Google Scholar] [CrossRef]
- Wang, P.; Meng, Q.; Wang, W.; Zhang, S.; Xiong, X.; Qin, S.; Zhang, J.; Li, A.; Liu, Z. Icariin inhibits the inflammation through down-regulating NF-κB/HIF-2α signal pathways in chondrocytes. Biosci. Rep. 2020, 40, BSR20203107. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.A.; Chen, C.-M.; Guan, S.-S.; Chiang, C.-K.; Wu, C.-T.; Liu, S.-H. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine 2019, 59, 152917. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, Z.; Hashemzadeh, M.R.; Colagar, A.H.; Jaafari, M.R. Signal transducer and activator of transcription 3 downregulation in J774A.1 cell line as a model of M2 macrophages in tumor microenvironment. J. Cancer Res. Ther. 2018, 14, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Baghdadi, M.; Chiba, S.; Yoshiyama, H. Regulation of cancer stem cell activities by tumor-associated macrophages. Am. J. Cancer Res. 2012, 2, 529–539. [Google Scholar]
- Aramini, B.; Masciale, V.; Grisendi, G.; Banchelli, F.; D’Amico, R.; Maiorana, A.; Morandi, U.; Dominici, M.; Haider, K.H. Cancer stem cells and macrophages: Molecular connections and future perspectives against cancer. Oncotarget 2021, 12, 230–250. [Google Scholar] [CrossRef]





| Identification | |
| Chemical Names | Icariin |
| Molecular Formula | C34H42O14 |
| Lipinski rules component | |
| Molecular Weight | 674.699 |
| logP | 2.682 |
| Hydrogen bond acceptors (HBA) | 11 |
| Hydrogen bond donors (HBD) | 7 |
| Matching Lipinski Rules | 1 |
| Veber rules component | |
| Polar Surface Area (PSA) | 214.06 |
| Rotatable Bond (RotB) | 10 |
| Matching Veber Rules | 0 |
| Scope | Applications | Ref. |
|---|---|---|
| Bone tissue engineering |
| [112] |
| [113] | |
| [114] | |
| [115] | |
| [128] | |
| Cartilage tissue engineering |
| [122] |
Compared with hyaluronic acid/collagen (HA/Col) hydrogel, ICRN-HA/Col hydrogel:
| [123] | |
1 × 10−6 M concentration of ICRN in differentiation medium of BMSCs
| [129] | |
In the model of osteoarthritis (OA) injected by HA/Poloxamer 407/ICRN hydrogel:
| [130] | |
| [125] |
| Scope | Result | Ref |
|---|---|---|
| Neuroprotective |
| [168] |
| [169] | |
| [170] | |
| Cardiovascular diseases |
| [171] |
| [172] | |
| [173] | |
| Reproductive system |
| [174] |
| [175] | |
| [176] | |
| Anti-tumor |
| [177] |
| [178] | |
| [179] | |
| Anti-inflammation |
| [180] |
| [181] | |
| [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyedi, Z.; Amiri, M.S.; Mohammadzadeh, V.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Mashreghi, M.; Qayoomian, M.; Hashemzadeh, M.R.; Simal-Gandara, J.; Taghavizadeh Yazdi, M.E. Icariin: A Promising Natural Product in Biomedicine and Tissue Engineering. J. Funct. Biomater. 2023, 14, 44. https://doi.org/10.3390/jfb14010044
Seyedi Z, Amiri MS, Mohammadzadeh V, Hashemzadeh A, Haddad-Mashadrizeh A, Mashreghi M, Qayoomian M, Hashemzadeh MR, Simal-Gandara J, Taghavizadeh Yazdi ME. Icariin: A Promising Natural Product in Biomedicine and Tissue Engineering. Journal of Functional Biomaterials. 2023; 14(1):44. https://doi.org/10.3390/jfb14010044
Chicago/Turabian StyleSeyedi, Zahra, Mohammad Sadegh Amiri, Vahideh Mohammadzadeh, Alireza Hashemzadeh, Aliakbar Haddad-Mashadrizeh, Mohammad Mashreghi, Mohsen Qayoomian, Mohammad Reza Hashemzadeh, Jesus Simal-Gandara, and Mohammad Ehsan Taghavizadeh Yazdi. 2023. "Icariin: A Promising Natural Product in Biomedicine and Tissue Engineering" Journal of Functional Biomaterials 14, no. 1: 44. https://doi.org/10.3390/jfb14010044
APA StyleSeyedi, Z., Amiri, M. S., Mohammadzadeh, V., Hashemzadeh, A., Haddad-Mashadrizeh, A., Mashreghi, M., Qayoomian, M., Hashemzadeh, M. R., Simal-Gandara, J., & Taghavizadeh Yazdi, M. E. (2023). Icariin: A Promising Natural Product in Biomedicine and Tissue Engineering. Journal of Functional Biomaterials, 14(1), 44. https://doi.org/10.3390/jfb14010044