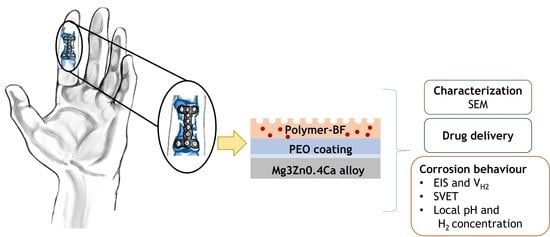
Ciprofloxacin Release and Corrosion Behaviour of a Hybrid PEO/PCL Coating on Mg3Zn0.4Ca Alloy
Abstract
:Highlights:
- Drug eluting hybrid coating comprising a PEO layer and porous polymer top-coat is successfully formed on Mg3Zn0.4Ca alloy.
- The hierarchical coating system avoids burst release and provides gradual elution of 64% of the drug load over 240 h of immersion.
- Ciprofloxacin loaded in the coating system shows a corrosion inhibiting effect of 74% compared to non-loaded specimen.
- Inhibition mechanism of ciprofloxacin involves the formation of insoluble metal chelates with (Ca, Mg) ions released during corrosion from the PEO layer and the substrate that seals the coating defects.
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Procedures
2.3. Surface Characterization
2.4. Drug Release
2.5. Corrosion Testing
2.5.1. Electrochemical Impedance Spectroscopy (EIS)
2.5.2. Hydrogen Evolution Test
2.5.3. Localized Measurements
3. Results and Discussion
3.1. Characterization of Functionalized Materials
3.2. Controlled Drug Release from Loaded Hybrid Hierarchical Coating
3.3. Corrosion Behaviour and Inhibitor Effect of Loaded Hybrid Ceramic-Polymeric Materials
3.3.1. Hydrogen Evolution Test
3.3.2. Localized Measurements (SVET, Local pH and Dissolved H2 Concentration)
3.3.3. Electrochemical Impedance Spectroscopy (EIS) and Post-Immersion Characterization
4. Conclusions
- The pore formation on the top-coat porous PCL layer is influenced only slightly by the presence of ciprofloxacin in the polymer/chloroform solution (i.e., pore diameter slightly increases).
- The drug-loaded HHC system avoided burst release and ensured gradual elution of the load over 240 h. The released ciprofloxacin load fraction from the HHC system on Mg3Zn0.4Ca alloy (64%) was lower than for the PCL film on a glass substrate (>80%) due the complexation of Mg(II) and Ca(II) cations released by the PEO coating and Mg substrate with ciprofloxacin.
- The drug-loaded HHC system presented overall higher and more stable corrosion protection with time than the drug-free system due to the precipitation of insoluble chelates that impeded the access of corrosive species of the medium through PCL and PEO layers of HHC reducing the degradation rate of Mg.
- Over 11 days of immersion in pseudo-physiological conditions (at constant pH 7.4 under CO2 flow), the CIP-loaded HHC system revealed 74% reduction of the degradation rate of Mg alloy compared to the drug-free one.
- Local current density distribution maps revealed an order of magnitude corrosion inhibiting effect of ciprofloxacin loaded into HHC-coated Mg3Zn0.4Ca alloy. The active corrosion protection mechanism involves the initial short-term acceleration of Mg corrosion due to the complexing of the released Mg2+ ions followed by the coating defect blocking action of the precipitated insoluble chelates of Mg2+ and Ca2+ cations with continuously released zwitterionic form of ciprofloxacin.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloys 2022, 10, 627–669. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Sánchez, H.M.; Moreno, L.; Arrabal, R.; Mohedano, M.; Gallardo, A.; Rodríguez-Hernández, J.; Matykina, E. Hybrid Functionalized Coatings on Metallic Biomaterials for Tissue Engineering. Surf. Coat. Technol. 2021, 422, 127508. [Google Scholar] [CrossRef]
- Mohedano, M.; Lu, X.; Matykina, E.; Blawert, C.; Arrabal, R.; Zheludkevich, M.L. Plasma Electrolytic Oxidation (PEO) of Metals and Alloys. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 423–438. ISBN 978-0-12-809894-3. [Google Scholar]
- Vaghefinazari, B.; Wang, C.; Mercier, D.; Mei, D.; Seyeux, A.; Marcus, P.; Blawert, C.; Lamaka, S.V.; Zheludkevich, M.L. Adverse Effect of 2,5PDC Corrosion Inhibitor on PEO Coated Magnesium. Corros. Sci. 2021, 192, 109830. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Lamaka, S.V.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Karlova, P.; Wieland, D.C.F.; Zheludkevich, M.L. Exploring the Corrosion Inhibition Mechanism of 8-Hydroxyquinoline for a PEO-Coated Magnesium Alloy. Corros. Sci. 2022, 203, 110344. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Onukwuli, O.D.; Abeng, F.E.; Okafor, N.A.; Ezeugo, J.O.; Okoye, C.C. Electrochemical-Kinetics, MD-Simulation and Multi-Input Single-Output (MISO) Modeling Using Adaptive Neuro-Fuzzy Inference System (ANFIS) Prediction for Dexamethasone Drug as Eco-Friendly Corrosion Inhibitor for Mild Steel in 2 M HCl Electrolyte. J. Taiwan Inst. Chem. Eng. 2020, 115, 251–265. [Google Scholar] [CrossRef]
- Akpan, I.A.; Offiong, N.-A.O. Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by Ciprofloxacin Drug. Int. J. Corros. 2013, 2013, e301689. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Agboola, O.; Ayeni, A.O. Corrosion Inhibitors as Building Evidence for Mild Steel: A Review. J. Phys. Conf. Ser. 2019, 1378, 032046. [Google Scholar] [CrossRef]
- Fekry, A.M.; Fatayerji, M.Z. Electrochemical Corrosion Behavior of AZ91D Alloy in Ethylene Glycol. Electrochim. Acta 2009, 54, 6522–6528. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Bakry, A.M. Role of Amoxicillin in Enhancing AZ31 Alloy Degradation Resistance and Its Monitoring Using Nano-Pd Electrochemical Sensor. Mater. Chem. Phys. 2019, 234, 224–236. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Tayebi, L. Development and Degradation Behavior of Magnesium Scaffolds Coated with Polycaprolactone for Bone Tissue Engineering. Mater. Lett. 2014, 132, 106–110. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M.; Rodríguez-Hernández, J. Towards Hierarchically Ordered Functional Porous Polymeric Surfaces Prepared by the Breath Figures Approach. Prog. Polym. Sci. 2014, 39, 510–554. [Google Scholar] [CrossRef] [Green Version]
- Daavari, M.; Atapour, M.; Mohedano, M.; Sánchez, H.M.; Rodríguez-Hernández, J.; Matykina, E.; Arrabal, R.; Taherizadeh, A. Quasi-In Vivo Corrosion Behavior of AZ31B Mg Alloy with Hybrid MWCNTs-PEO/PCL Based Coatings. J. Magnes. Alloys 2022, 10, 3217–3233. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Mei, D.; Deng, M.; Zheng, Y.; Zheludkevich, M.L.; Lamaka, S.V. Local PH and Oxygen Concentration at the Interface of Zn Alloys in Tris-HCl or HEPES Buffered Hanks’ Balanced Salt Solution. Corros. Sci. 2022, 197, 110061. [Google Scholar] [CrossRef]
- Wang, C.; Tonna, C.; Mei, D.; Buhagiar, J.; Zheludkevich, M.L.; Lamaka, S.V. Biodegradation Behaviour of Fe-Based Alloys in Hanks’ Balanced Salt Solutions: Part II. The Evolution of Local PH and Dissolved Oxygen Concentration at Metal Interface. Bioact. Mater. 2022, 7, 412–425. [Google Scholar] [CrossRef]
- Han, S.; Yang, R.; Li, C.; Yang, L. The Wettability and Numerical Model of Different Silicon Microstructural Surfaces. Appl. Sci. 2019, 9, 566. [Google Scholar] [CrossRef] [Green Version]
- Kłosińska-Szmurło, E.; Pluciński, F.A.; Grudzień, M.; Betlejewska-Kielak, K.; Biernacka, J.; Mazurek, A.P. Experimental and Theoretical Studies on the Molecular Properties of Ciprofloxacin, Norfloxacin, Pefloxacin, Sparfloxacin, and Gatifloxacin in Determining Bioavailability. J. Biol. Phys. 2014, 40, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walden, D.M.; Khotimchenko, M.; Hou, H.; Chakravarty, K.; Varshney, J. Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study. Pharmaceutics 2021, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Bridle, M.J.; Janesko, B.G. Computational Study of Fluoroquinolone Binding to and Its Applicability to Future Drug Design. Int. J. Quantum Chem. 2017, 117, e25428. [Google Scholar] [CrossRef]
- Refat, M.S.; Mohamed, G.G.; El-Sayed, M.Y.; Killa, H.M.A.; Fetooh, H. Spectroscopic and Thermal Degradation Behavior of Mg(II), Ca(II), Ba(II) and Sr(II) Complexes with Paracetamol Drug. Arab. J. Chem. 2017, 10, S2376–S2387. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Liu, Y.; Guo, M.; Fang, H.; Xu, X. Corrosion and Drug Release Properties of EN-Plating/PLGA Composite Coating on MAO Film. Mater. Sci. Eng. C 2011, 31, 1285–1289. [Google Scholar] [CrossRef]
- Xu, X.; Lu, P.; Guo, M.; Fang, M. Cross-Linked Gelatin/Nanoparticles Composite Coating on Micro-Arc Oxidation Film for Corrosion and Drug Release. Appl. Surf. Sci. 2010, 256, 2367–2371. [Google Scholar] [CrossRef]
- Ji, X.-J.; Gao, L.; Liu, J.-C.; Wang, J.; Cheng, Q.; Li, J.-P.; Li, S.-Q.; Zhi, K.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion Resistance and Antibacterial Properties of Hydroxyapatite Coating Induced by Gentamicin-Loaded Polymeric Multilayers on Magnesium Alloys. Colloids Surf. B Biointerfaces 2019, 179, 429–436. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Asakura, T.; Sasaki, M.; Niidome, T. Silk Fibroin as a Coating Polymer for Sirolimus-Eluting Magnesium Alloy Stents. ACS Appl. Bio Mater. 2020, 3, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eduok, U.M.; Umoren, S.; Udoh, A. Synergistic Inhibition Effects between Leaves and Stem Extracts of Sida Acuta and Iodide Ion for Mild Steel Corrosion in 1 M H2SO4 Solutions. Arab. J. Chem. 2012, 5, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Srinivasulu, A.; Kasthuri, P. Study of Inhibition and Adsorption Properties of Mild Steel Corrosion by Expired Pharmaceutical Gentamicin Drug in Hydrochloric Acid Media. Orient. J. Chem. 2017, 33, 2616–2624. [Google Scholar] [CrossRef]
- Palacio, D.A.; Rivas, B.L.; Urbano, B.F. Ultrafiltration Membranes with Three Water-Soluble Polyelectrolyte Copolymers to Remove Ciprofloxacin from Aqueous Systems. Chem. Eng. J. 2018, 351, 85–93. [Google Scholar] [CrossRef]
- Yang, L.; Qin, X.; Jiang, X.; Gong, M.; Yin, D.; Zhang, Y.; Zhao, B. SERS Investigation of Ciprofloxacin Drug Molecules on TiO2 Nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 17809–17815. [Google Scholar] [CrossRef]
- Palafox, M.; Rastogi, V. Spectra and Structure of Benzonitriles and Some of Its Simple Derivatives Spectra and Structure of Benzonitriles and Some of Its Simple Derivatives. Asian J. Phys. 2013, 22, 43–73. [Google Scholar]
- Massoumi, B.; Ramezani, M.; Jaymand, M.; Ahmadinejad, M. Multi-Walled Carbon Nanotubes-g-[Poly(Ethylene Glycol)-b-Poly(ε-Caprolactone)]: Synthesis, Characterization, and Properties. J. Polym. Res. 2015, 22, 214. [Google Scholar] [CrossRef]
- Chandrathilaka, A.; Ileperuma, O.; Hettiarachchi, C. Spectrophotometric and PH-Metric Studies on Pb(II), Cd(II), Al(III) and Cu(II) Complexes of Paracetamol and Ascorbic Acid. J. Natl. Sci. Found. Sri Lanka 2013, 41, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.L.; Matykina, E. PEO Coatings Design for Mg-Ca Alloy for Cardiovascular Stent and Bone Regeneration Applications. Mater. Sci. Eng. C 2019, 105, 110026. [Google Scholar] [CrossRef] [PubMed]













| HHC System | Pore Size (µm) | Surface Pore Population Density (Pore/mm2)· 103 | Contact Angle (º) | Sa (µm) | S10z (µm) |
|---|---|---|---|---|---|
| HHC | 8.5 ± 2.8 | 298 | 88.9 ± 5.0 | 0.6 ± 0.2 | 7.2 ± 3.7 |
| HHC+CIP | 12.2 ± 1.1 | 76 | 96.1 ± 3.0 | 0.5 ± 0.1 | 5.2 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, L.; Wang, C.; Lamaka, S.V.; Zheludkevich, M.L.; Rodríguez-Hernández, J.; Arrabal, R.; Matykina, E. Ciprofloxacin Release and Corrosion Behaviour of a Hybrid PEO/PCL Coating on Mg3Zn0.4Ca Alloy. J. Funct. Biomater. 2023, 14, 65. https://doi.org/10.3390/jfb14020065
Moreno L, Wang C, Lamaka SV, Zheludkevich ML, Rodríguez-Hernández J, Arrabal R, Matykina E. Ciprofloxacin Release and Corrosion Behaviour of a Hybrid PEO/PCL Coating on Mg3Zn0.4Ca Alloy. Journal of Functional Biomaterials. 2023; 14(2):65. https://doi.org/10.3390/jfb14020065
Chicago/Turabian StyleMoreno, Lara, Cheng Wang, Sviatlana V. Lamaka, Mikhail L. Zheludkevich, Juan Rodríguez-Hernández, Raul Arrabal, and Endzhe Matykina. 2023. "Ciprofloxacin Release and Corrosion Behaviour of a Hybrid PEO/PCL Coating on Mg3Zn0.4Ca Alloy" Journal of Functional Biomaterials 14, no. 2: 65. https://doi.org/10.3390/jfb14020065
APA StyleMoreno, L., Wang, C., Lamaka, S. V., Zheludkevich, M. L., Rodríguez-Hernández, J., Arrabal, R., & Matykina, E. (2023). Ciprofloxacin Release and Corrosion Behaviour of a Hybrid PEO/PCL Coating on Mg3Zn0.4Ca Alloy. Journal of Functional Biomaterials, 14(2), 65. https://doi.org/10.3390/jfb14020065