Functionalization of a Cortical Membrane with a Photodynamic Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Membrane
2.1.2. ALAD-PDT Protocol
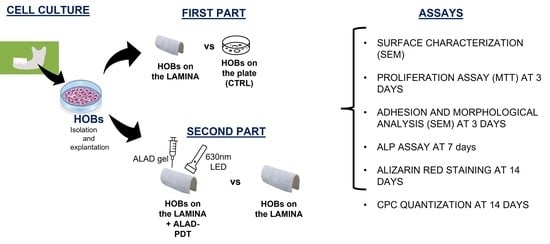
2.2. Experimental Design
- -
- In TEST 1, the response of human oral osteoblasts (HOBs) was evaluated for lamina compared to ones seeded on a well/plate. Two groups were compared, HOBs seeded on the plate as control (CTRL) and HOBs seeded on the membrane (lamina). SEM analyses were performed to study the topographical characteristics of the membrane surface and the adhesion and morphology of cells at 3 days. The viability was assessed at 3 days by MTT assay, the ALP activity was investigated at 7 days by ALP enzymatic assay, and calcium deposition at 14 days was assessed by Alizarin Red staining and by the measurement of absorbance;
- -
- In TEST 2, the activity of HOBs seeded on the lamina was compared to the response of HOBs seeded on the lamina surface and then subjected to the ALAD-PDT protocol.
- -
- HOBs seeded on the membrane (lamina);
- -
- HOBs seeded on the membrane and treated with ALAD gel for 45 min (ALAD);
- -
- HOBs cultured on the membrane and irradiated with 630 nm LED for 7 min (PDT);
- -
- HOBs cultured on the membrane, treated with ALAD gel, and irradiated with 630 nm LED for 7 min (ALAD-PDT).
2.3. Characterization of the Lamina
2.4. Biological In Vitro Tests
2.4.1. Cell Culture
2.4.2. MTT Assay
2.4.3. Cell Adhesion and Morphology
2.4.4. ALP Assay
2.4.5. Alizarin Red Staining and Quantification of Calcium Deposition
2.4.6. Statistical Analysis
3. Results
3.1. Characterization of the Lamina
3.2. Biological In Vitro TEST 1
3.2.1. Cell Viability
3.2.2. Cell Adhesion
3.2.3. ALP Activity
3.2.4. Mineralization
3.3. Biological In Vitro TEST 2
3.3.1. Cell Viability
3.3.2. Adhesion of Osteoblasts on the Lamina
3.3.3. ALP Activity
3.3.4. Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed]
- Stájer, A.; Kajári, S.; Gajdács, M.; Musah-Eroje, A.; Baráth, Z. Utility of Photodynamic Therapy in Dentistry: Current Concepts. Dent. J. 2020, 8, 43. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, J.; Bu, W.; Zhu, X.; Zhang, P.; Zhu, Y.; Fan, X.; Wang, C. Targeted Implementation Strategies of Precise Photodynamic Therapy Based on Clinical and Technical Demands. Biomater. Sci. 2022, 11, 704–718. [Google Scholar] [CrossRef]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J.; Shuin, T. The Inhibition of Ferrochelatase Enhances 5-Aminolevulinic Acid-Based Photodynamic Action for Prostate Cancer. Photodiagnosis Photodyn. Ther. 2013, 10, 399–409. [Google Scholar] [CrossRef]
- Ohgari, Y.; Nakayasu, Y.; Kitajima, S.; Sawamoto, M.; Mori, H.; Shimokawa, O.; Matsui, H.; Taketani, S. Mechanisms Involved in Delta-Aminolevulinic Acid (ALA)-Induced Photosensitivity of Tumor Cells: Relation of Ferrochelatase and Uptake of ALA to the Accumulation of Protoporphyrin. Biochem. Pharmacol. 2005, 71, 42–49. [Google Scholar] [CrossRef]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The Good, the Bad, and the Ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef]
- Li, X.; Guo, H.; Tian, Q.; Zheng, G.; Hu, Y.; Fu, Y.; Tan, H. Effects of 5-Aminolevulinic Acid-Mediated Photodynamic Therapy on Antibiotic-Resistant Staphylococcal Biofilm: An in Vitro Study. J. Surg. Res. 2013, 184, 1013–1021. [Google Scholar] [CrossRef]
- Nakai, Y.; Tatsumi, Y.; Miyake, M.; Anai, S.; Kuwada, M.; Onishi, S.; Chihara, Y.; Tanaka, N.; Hirao, Y.; Fujimoto, K. Expression of Ferrochelatase Has a Strong Correlation in Protoporphyrin IX Accumulation with Photodynamic Detection of Bladder Cancer. Photodiagnosis Photodyn. Ther. 2016, 13, 225–232. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a Novel Gel Containing 5-Aminolevulinic Acid and Red LED against Bacteria Involved in Peri-Implantitis and Other Oral Infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef]
- Petrini, M.; di Lodovico, S.; Iezzi, G.; Cellini, L.; Tripodi, D.; Piattelli, A.; D’ercole, S. Photodynamic Antibiofilm and Antibacterial Activity of a New Gel with 5-Aminolevulinic Acid on Infected Titanium Surfaces. Biomedicines 2022, 10, 572. [Google Scholar] [CrossRef]
- di Lodovico, S.; Diban, F.; di Fermo, P.; Petrini, M.; Fontana, A.; di Giulio, M.; Piattelli, A.; D’ercole, S.; Cellini, L. Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management. Int. J. Mol. Sci. 2022, 23, 6942. [Google Scholar] [CrossRef]
- Rossi, R.; Rispoli, L.; Lopez, M.A.; Netti, A.; Petrini, M.; Piattelli, A. Photodynamic Therapy by Mean of 5-Aminolevulinic Acid for the Management of Periodontitis and Peri-Implantitis: A Retrospective Analysis of 20 Patients. Antibiotics 2022, 11, 1267. [Google Scholar] [CrossRef]
- D’Ercole, S.; Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Tripodi, D.; Iezzi, G.; Piattelli, A.; Petrini, M. 5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report. Gels 2022, 8, 697. [Google Scholar] [CrossRef]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; Carlesi, T.; Iezzi, G.; D’arcangelo, C.; di Lodovico, S.; Piattelli, A.; D’ercole, S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus Faecalis and Human Gingival Fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 3048. [Google Scholar] [CrossRef]
- Pierfelice, T.V.; D’Amico, E.; Iezzi, G.; Petrini, M.; Schiavone, V.; Santalucia, M.; Pandolfi, A.; D’Arcangelo, C.; Piattelli, A.; di Pietro, N. Effect of a 5-Aminolevulinic Acid Gel and 660 Nm Red LED Light on Human Oral Osteoblasts: A Preliminary in Vitro Study. Lasers Med. Sci. 2022, 37, 3671–3679. [Google Scholar] [CrossRef]
- Pierfelice, T.V.; D’Amico, E.; Petrini, M.; Pandolfi, A.; D’Arcangelo, C.; di Pietro, N.; Piattelli, A.; Iezzi, G. The Effects of 5% 5-Aminolevulinic Acid Gel and Red Light (ALAD-PDT) on Human Fibroblasts and Osteoblasts. Gels 2022, 8, 491. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical Classification of Complications in Guided Bone Regeneration Procedures by Means of a Non Resorbable Membrane. Int. J. Periodontics Restor. Dent. 2011, 3, 265–273. [Google Scholar]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-Collagen-Hydroxyapatite Membranes for Tissue Engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Villagrán, M.; Leger, D.Y.; Liagre, B.; Therrien, B. Photosensitizers Used in the Photodynamic Therapy of Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 3339. [Google Scholar] [CrossRef]
- Gholami, L.; Shahabi, S.; Jazaeri, M.; Hadilou, M.; Fekrazad, R. Clinical Applications of Antimicrobial Photodynamic Therapy in Dentistry. Front. Microbiol. 2023, 13, 1020995. [Google Scholar] [CrossRef]
- Ramos, U.D.; Suaid, F.A.; Wikesjö, U.M.E.; Susin, C.; Taba, M.; Novaes, A.B. Comparison between Two Antimicrobial Protocols with or without Guided Bone Regeneration in the Treatment of Peri-Implantitis. A Histomorphometric Study in Dogs. Clin. Oral Implant. Res. 2017, 28, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Carvalho, G.; Sanchez-Puetate, J.C.; Casalle, N.; Marcantonio Junior, E.; Leal Zandim-Barcelos, D. Antimicrobial Photodynamic Therapy Associated with Bone Regeneration for Peri-Implantitis Treatment: A Case Report. Photodiagnosis Photodyn. Ther. 2020, 30, 101705. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, L. T-Test and ANOVA for Data with Ceiling and/or Floor Effects. Behav. Res. Methods 2021, 53, 264–277. [Google Scholar] [CrossRef] [PubMed]
- di Carlo, R.; Zara, S.; Ventrella, A.; Siani, G.; da Ros, T.; Iezzi, G.; Cataldi, A.; Fontana, A. Covalent Decoration of Cortical Membranes with Graphene Oxide as a Substrate for Dental Pulp Stem Cells. Nanomaterials 2019, 9, 604. [Google Scholar] [CrossRef]
- Wachtel, H.; Fickl, S.; Hinze, M.; Bolz, W.; Thalmair, T. The Bone Lamina Technique: A Novel Approach for Lateral Ridge Augmentation--a Case Series. Int. J. Periodontics Restor. Dent. 2013, 33, 491–497. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The Effect of Pore Size on Cell Adhesion in Collagen-GAG Scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; InTech Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, J.; Yu, Y.; Rong, M.; Yang, T. A Rapid and Convenient Approach to Construct Porous Collagen Membranes via Bioskiving and Sonication-Feasible for Mineralization to Induce Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 657357. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Palmieri, A.; della Vella, F.; Petruzzi, M.; Botticelli, D.; Carinci, F. Photodynamic Therapy Using 5-Aminolevulinic Acid (Ala) for the Treatment of Chronic Periodontitis: A Prospective Case Series. Appl. Sci. 2022, 12, 3102. [Google Scholar] [CrossRef]
- Azaripour, A.; Azaripour, M.; Willershausen, I.; van Noorden, C.J.F.; Willershausen, B. Photodynamic Therapy Has No Adverse Effects In Vitro on Human Gingival Fibroblasts and Osteoblasts. Clin. Lab. 2018, 64, 1225–1231. [Google Scholar] [CrossRef]
- Ou, J.; Gao, Y.; Li, H.; Ling, T.; Xie, X. Application of 5-Aminolevulinic Acid-Mediated Waterlase-Assisted Photodynamic Therapy in the Treatment of Oral Leukoplakia. Sci. Rep. 2022, 12, 9391. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, Y.; Zhiyue, L. Successful Treatment of Oral Human Papilloma by Local Injection 5-Aminolevulinic Acid-Mediated Photodynamic Therapy: A Case Report. Photodiagnosis Photodyn. Ther. 2019, 26, 134–136. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierfelice, T.V.; D’Amico, E.; D’Ercole, S.; Lepore, S.; Piattelli, A.; Barone, A.; Iezzi, G.; Petrini, M. Functionalization of a Cortical Membrane with a Photodynamic Protocol. J. Funct. Biomater. 2023, 14, 133. https://doi.org/10.3390/jfb14030133
Pierfelice TV, D’Amico E, D’Ercole S, Lepore S, Piattelli A, Barone A, Iezzi G, Petrini M. Functionalization of a Cortical Membrane with a Photodynamic Protocol. Journal of Functional Biomaterials. 2023; 14(3):133. https://doi.org/10.3390/jfb14030133
Chicago/Turabian StylePierfelice, Tania Vanessa, Emira D’Amico, Simonetta D’Ercole, Stefania Lepore, Adriano Piattelli, Antonio Barone, Giovanna Iezzi, and Morena Petrini. 2023. "Functionalization of a Cortical Membrane with a Photodynamic Protocol" Journal of Functional Biomaterials 14, no. 3: 133. https://doi.org/10.3390/jfb14030133
APA StylePierfelice, T. V., D’Amico, E., D’Ercole, S., Lepore, S., Piattelli, A., Barone, A., Iezzi, G., & Petrini, M. (2023). Functionalization of a Cortical Membrane with a Photodynamic Protocol. Journal of Functional Biomaterials, 14(3), 133. https://doi.org/10.3390/jfb14030133