Adipogenesis-Related Metabolic Condition Affects Shear-Stressed Endothelial Cells Activity Responding to Titanium
Abstract
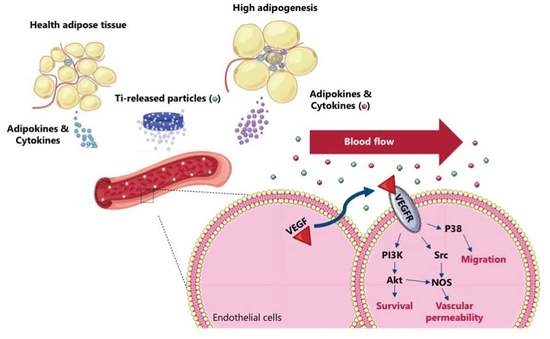
:1. Introduction
2. Methods
2.1. Implants
2.2. Reagents
2.3. Cell Culture
2.4. Adipocyte Differentiation
2.5. Shear Stress Model
2.6. Cell Viability Assay
2.7. Oil Red O Staining
2.8. Titanium-Enriched Medium Obtaining
2.9. Western Blot
2.10. Quantitative PCR Assay (qPCR)
2.11. Oxidative Stress Markers
2.12. Matrix Metalloproteinases (MMPs) Activities by Zymography
2.13. Statistical Analysis
3. Results
3.1. Validation of the High-Adipogenesis Model
3.2. Angiogenesis-Related Genes Were Evaluated in ECs Responding to High Adipogenesis and Titanium
3.3. Proliferation and Survival-Related Genes in ECs
3.4. Endothelial Cell Appears to Be Important in Inflammatory Gene Microenvironment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, S.M.; O’Connell, B.P.; Meyer, T.A. Obesity related complications in surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 341–347. [Google Scholar] [CrossRef]
- Adamczak, M.; Wiecek, A. The adipose tissue as an endocrine organ. Semin. Nephrol. 2013, 33, 2–13. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuche, C. The cardiovascular system and associated disorders. Br. J. Nurs. 2022, 31, 886–892. [Google Scholar] [CrossRef]
- Givens, C.; Tzima, E. Endothelial Mechanosignaling: Does One Sensor Fit All? Antioxid. Redox Signal. 2016, 25, 373–388. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.A.; Ferreira, M.R.; Gomes, A.M.; Zambuzzi, W.F. LncRNA HOTAIR is a novel endothelial mechanosensitive gene. J. Cell. Physiol. 2020, 235, 4631–4642. [Google Scholar] [CrossRef]
- Gomes, A.M.; Pinto, T.S.; da Costa Fernandes, C.J.; da Silva, R.A.; Zambuzzi, W.F. Wortmannin targeting phosphatidylinositol 3-kinase suppresses angiogenic factors in shear-stressed endothelial cells. J. Cell. Physiol. 2020, 235, 5256–5269. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.A.; Fernandes, C.J.d.C.; Feltran, G.d.S.; Gomes, A.M.; de Camargo Andrade, A.F.; Andia, D.C.; Peppelenbosch, M.P.; Zambuzzi, W.F. Laminar shear stress-provoked cytoskeletal changes are mediated by epigenetic reprogramming of TIMP1 in human primary smooth muscle cells. J. Cell. Physiol. 2019, 234, 6382–6396. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, B.R.; Pinto, T.S.; da Costa Fernandes, C.J.; Bezerra, F.; Zambuzzi, W.F. PI3K/AKT signaling drives titanium-induced angiogenic stimulus. J. Mater. Sci. Mater. Med. 2021, 32, 18. [Google Scholar] [CrossRef]
- Pinto, T.S.; Martins, B.R.; Ferreira, M.R.; Bezerra, F.; Zambuzzi, W.F. Nanohydroxyapatite-Blasted Bioactive Surface Drives Shear-Stressed Endothelial Cell Growth and Angiogenesis. BioMed Res. Int. 2022, 2022, 1433221. [Google Scholar] [CrossRef]
- Chen, W.; Xu, K.; Tao, B.; Dai, L.; Yu, Y.; Mu, C.; Shen, X.; Hu, Y.; He, Y.; Cai, K. Multilayered coating of titanium implants promotes coupled osteogenesis and angiogenesis in vitro and in vivo. Acta Biomater. 2018, 74, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.F.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, T.; Horvath, J.M.; van Blitterswijk, C.A.; LaPointe, V.L.S. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J. Tissue Eng. Regen. Med. 2019, 13, 1815–1829. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Pippenger, B.; Tovar, N.; Koopmans, S.-J.; Plana, N.M.; Graves, D.T.; Engebretson, S.; van Beusekom, H.M.M.; Oliveira, P.G.F.P.; Dard, M. Effect of Obesity or Metabolic Syndrome and Diabetes on Osseointegration of Dental Implants in a Miniature Swine Model: A Pilot Study. J. Oral Maxillofac. Surg. 2018, 76, 1677–1687. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Gigante, I.; Colucci, S.; Grano, M. Periodontal disease: Linking the primary inflammation to bone loss. Clin. Dev. Immunol. 2013, 2013, 503754. [Google Scholar] [CrossRef] [Green Version]
- Gottlander, M.; Johansson, C.B.; Wennerberg, A.; Albrektsson, T.; Radin, S.; Ducheyne, P. Bone tissue reactions to an electrophoretically applied calcium phosphate coating. Biomaterials 1997, 18, 551–557. [Google Scholar] [CrossRef]
- Meirelles, L.; Arvidsson, A.; Andersson, M.; Kjellin, P.; Albrektsson, T.; Wennerberg, A. Nano hydroxyapatite structures influence early bone formation. J. Biomed. Mater. Res. A 2008, 87, 299–307. [Google Scholar] [CrossRef]
- dela Paz, N.G.; Walshe, T.E.; Leach, L.L.; Saint-Geniez, M.; D’Amore, P.A. Role of shear-stress-induced VEGF expression in endothelial cell survival. J. Cell Sci. 2012, 125, 831–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, T.S.; Fernandes, C.J.d.C.; da Silva, R.A.; Gomes, A.M.; Vieira, J.C.S.; Padilha, P.d.M.; Zambuzzi, W.F. c-Src kinase contributes on endothelial cells mechanotransduction in a heat shock protein 70-dependent turnover manner. J. Cell. Physiol. 2019, 234, 11287–11303. [Google Scholar] [CrossRef]
- Zambuzzi, W.F.; Bonfante, E.A.; Jimbo, R.; Hayashi, M.; Andersson, M.; Alves, G.; Takamori, E.R.; Beltrao, P.J.; Coelho, P.G.; Granjeiro, J.M. Nanometer scale titanium surface texturing are detected by signaling pathways involving transient FAK and Src activations. PLoS ONE 2014, 9, e95662. [Google Scholar] [CrossRef]
- Machado, M.I.P.; Gomes, A.M.; Rodrigues, M.F.; Silva Pinto, T.; da Costa Fernandes, C.J.; Bezerra, F.J.; Zambuzzi, W.F. Cobalt-chromium-enriched medium ameliorates shear-stressed endothelial cell performance. J. Trace Elem. Med. Biol. 2019, 54, 163–171. [Google Scholar] [CrossRef]
- da Costa Fernandes, C.J.; Bezerra, F.J.B.; de Campos Souza, B.; Campos, M.A.; Zambuzzi, W.F. Titanium-enriched medium drives low profile of ECM remodeling as a pre-requisite to pre-osteoblast viability and proliferative phenotype. J. Trace Elem. Med. Biol. 2018, 50, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, C.J.C.; Bezerra, F.; das D do Carmo, M.; Feltran, G.S.; Rossi, M.C.; da Silva, R.A.; Padilha, P.; Zambuzzi, W. CoCr-enriched medium modulates integrin-based downstream signaling and requires a set of inflammatory genes reprograming in vitro. J. Biomed. Mater. Res. A 2018, 106, 839–849. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Peeters-Joris, C.; Vaes, G. Production of gelatin-degrading matrix metalloproteinases (‘type IV collagenases’) and inhibitors by articular chondrocytes during their dedifferentiation by serial subcultures and under stimulation by interleukin-1 and tumor necrosis factor alpha. Biochim. Biophys. Acta 1991, 1094, 8–18. [Google Scholar] [CrossRef]
- Bezerra, F.; Ferreira, M.R.; Fontes, G.N.; da Costa Fernandes, C.J.; Andia, D.C.; Cruz, N.C.; da Silva, R.A.; Zambuzzi, W.F. Nano hydroxyapatite-blasted titanium surface affects pre-osteoblast morphology by modulating critical intracellular pathways. Biotechnol. Bioeng. 2017, 114, 1888–1898. [Google Scholar] [CrossRef]
- Fernandes, C.J.C.; Bezerra, F.; Ferreira, M.R.; Andrade, A.F.C.; Pinto, T.S.; Zambuzzi, W.F. Nano hydroxyapatite-blasted titanium surface creates a biointerface able to govern Src-dependent osteoblast metabolism as prerequisite to ECM remodeling. Colloids Surf. B Biointerfaces 2018, 163, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios-Ortega, S.; Varela-Guruceaga, M.; Martínez, J.A.; de Miguel, C.; Milagro, F.I. Effects of high glucose on caveolin-1 and insulin signaling in 3T3-L1 adipocytes. Adipocyte 2016, 5, 65–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.C.; Yang, R.S.; Tsai, K.S.; Ho, F.M.; Liu, S.H. Hyperglycemia Enhances Adipogenic Induction of Lipid Accumulation: Involvement of Extracellular Signal-Regulated Protein Kinase 1/2, Phosphoinositide 3-Kinase/Akt, and Peroxisome Proliferator-Activated Receptor γ Signaling. Endocrinology 2007, 148, 4267–4275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilpa, K.; Dinesh, T.; Lakshmi, B.S. An In Vitro Model to Probe the Regulation of Adipocyte Differentiation under Hyperglycemia. Diabetes Metab. J. 2013, 37, 176–180. [Google Scholar] [CrossRef] [Green Version]
- von Wilmowsky, C.; Stockmann, P.; Harsch, I.; Amann, K.; Metzler, P.; Lutz, R.; Moest, T.; Neukam, F.W.; Schlegel, K.A. Diabetes mellitus negatively affects peri-implant bone formation in the diabetic domestic pig. J. Clin. Periodontol. 2011, 38, 771–779. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, S.K.; Kusumbe, A.P.; Schiller, M.; Zeuschner, D.; Bixel, M.G.; Milia, C.; Gamrekelashvili, J.; Limbourg, A.; Medvinsky, A.; Santoro, M.M.; et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 2016, 7, 13601. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, P.G.F.P.; Bonfante, E.A.; Bergamo, E.T.P.; de Souza, S.L.S.; Riella, L.; Torroni, A.; Benalcazar Jalkh, E.B.; Witek, L.; Lopez, C.D.; Zambuzzi, W.F.; et al. Obesity/Metabolic Syndrome and Diabetes Mellitus on Peri-implantitis. Trends Endocrinol. Metab. 2020, 31, 596–610. [Google Scholar] [CrossRef]
- Zhang, X.; Simons, M. Receptor tyrosine kinases endocytosis in endothelium: Biology and signaling. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1831–1837. [Google Scholar] [CrossRef] [Green Version]
- Sonomoto, K.; Yamaoka, K.; Oshita, K.; Fukuyo, S.; Zhang, X.; Nakano, K.; Okada, Y.; Tanaka, Y. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 2012, 64, 3355–3363. [Google Scholar] [CrossRef] [PubMed]









| Genes | Primers | 5′-3′ Sequences | Reaction’s Conditions |
|---|---|---|---|
| H-AKT | Forward | CAG CGC GGC CCG AAG GAC | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | GAC GCT CAC GCG CTC CTC TC | ||
| H-CDK2 | Forward | CTT TGC TGA GAT GGT GAC TCG | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | GCC TCC CAG ATT CCT CAT GC | ||
| H-CDK4 | Forward | CTC TCT AGC TTG CGG CCT G | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | GCA GGG ATA CAT CTC GAG GC | ||
| H-ERK | Forward | GCA GCG CCT CCC TTG CTA GA | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | AAC AGC CTC TGG CCC ACC CAT | ||
| H-IL1B | Forward | GGA GAA TGA CCT GAG CAC CT | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | GGA GGT GGA GAG CTT TCA GT | ||
| H-IL6 | Forward | AGT CCT GAT CCA GTT CCT GC | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | CTA CAT TTG CCG AAG AGC CC | ||
| H-JNK | Forward | AAA GGT GGT GTT TTG TTC CCA GGT | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | TGA TGA TGG ATG CTG AGA GCC ATT G | ||
| H-P38 | Forward | GAG AAC TGC GGT TAC TTA | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | ATG GGT CAC CAG ATA CAC AT | ||
| H-VEGF | Forward | TGC AGA TTA TGC GGA TCA AAC C | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | TGC ATT CAC ATT TGT TGT GCT GTA G | ||
| H-VEGFr1 | Forward | CAG GCC CAG TTT CTG CCA TT | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | TTC CAG CTC AGC GTG GTC GTA | ||
| M-Gapdh | Forward | AGG CCG GTG CTG AGT ATG TC | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | TGC CTG CTT CAC CAC CTT CT | ||
| M-Il13 | Forward | CAG TCC TGG CTC TTG CTT G | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | CCA GGT CCA CAC TCC ATA CC | ||
| M-Il18 | Forward | ACT TTG GCC GAC TTC ACT GT | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | GGG TTC ACT GGC ACT TTG AT | ||
| M-Il1b | Forward | GAC CTT CCA GGA TGA GGA CA | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | AGC TCA TAT GGG TCC GAC AG | ||
| M-Il1r | Forward | ACC CCC ATA TCA GCG GAG CG | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | TTG CTT CCC CCG GAA CGT AT | ||
| M-Il33 | Forward | CCT TCT CGC TGA TTT CCA AG | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | CCG TTA CGG ATA TGG TGG TC | ||
| M-Il6 | Forward | AGT TGC CTT CTT GGG ACT GA | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | CAG AAT TGC CAT TGC ACA AC | ||
| M-Myd88 | Forward | ATG GTG GTG GTT GTT TCT GAC GA | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | GCA AGG GTT GGT ATA GTC GCA TAT A | ||
| M-Nfkb | Forward | CAC CTG TTC CAA AGA GCA CC | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | GGT TCA GGA GCT GCT GAA AC | ||
| M-Pparg | Forward | TTT TCA AGG GTG CCA GTT TC | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | AAT CCT TGG CCC TCT GAG AT | ||
| M-Tnf | Forward | CCA CAT CTC CCT CCA GAA AA | 95 °C, 3 s; 55 °C, 8 s; 72 °C, 20 s |
| Reverse | AGG GTC TGG GCC ATA GAA CT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, T.S.; Gomes, A.M.; de Morais, P.B.; Zambuzzi, W.F. Adipogenesis-Related Metabolic Condition Affects Shear-Stressed Endothelial Cells Activity Responding to Titanium. J. Funct. Biomater. 2023, 14, 162. https://doi.org/10.3390/jfb14030162
Pinto TS, Gomes AM, de Morais PB, Zambuzzi WF. Adipogenesis-Related Metabolic Condition Affects Shear-Stressed Endothelial Cells Activity Responding to Titanium. Journal of Functional Biomaterials. 2023; 14(3):162. https://doi.org/10.3390/jfb14030162
Chicago/Turabian StylePinto, Thaís Silva, Anderson Moreira Gomes, Paula Bertin de Morais, and Willian F. Zambuzzi. 2023. "Adipogenesis-Related Metabolic Condition Affects Shear-Stressed Endothelial Cells Activity Responding to Titanium" Journal of Functional Biomaterials 14, no. 3: 162. https://doi.org/10.3390/jfb14030162
APA StylePinto, T. S., Gomes, A. M., de Morais, P. B., & Zambuzzi, W. F. (2023). Adipogenesis-Related Metabolic Condition Affects Shear-Stressed Endothelial Cells Activity Responding to Titanium. Journal of Functional Biomaterials, 14(3), 162. https://doi.org/10.3390/jfb14030162