Zinc Oxide–Incorporated Chitosan–Poly(methacrylic Acid) Polyelectrolyte Complex as a Wound Healing Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
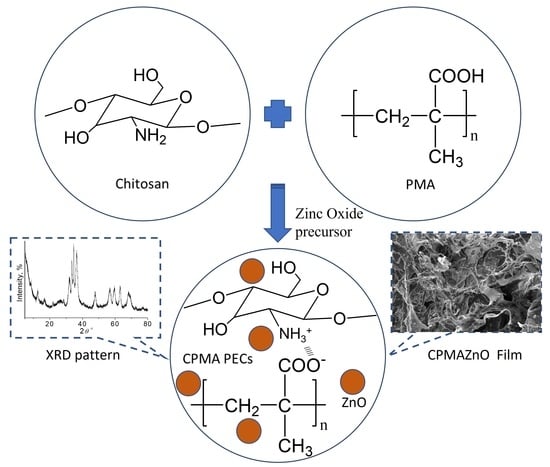
2.2. Synthesis
2.2.1. Preparation of PMA
2.2.2. Preparation of CPMA and CPMAZnO Films
2.2.3. Characterization Methods
2.3. Swelling Studies
2.4. In Vitro Biodegradation Studies
2.5. Antibacterial Studies
2.6. MTT and Live–Dead Cell Assay
3. Results and Discussion
3.1. Preparation and Characterization of CPMAZnO Films
3.2. Morphology and Porosity of CPMAZnO Films
3.3. Swelling Behavior of CPMAZnO Films
3.4. Tensile Strength and Biodegradation Studies
3.5. Antibacterial and Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Balagangadharan, K.; Selvamurugan, N.; Sathya Seeli, D.; Prabaharan, M. Preparation and characterization of chitosan/carboxymethyl pullulan/bioglass composite films for wound healing. J. Biomat. Appl. 2022, 36, 1151–1163. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Balagangadharan, K.; Selvamurugan, N.; Prabaharan, M. Porous wound dressings based on chitosan/carboxymethyl guar gum/TiO2 nanoparticles. AIP Conf. Proc. 2020, 2297, 020029. [Google Scholar]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, Y.; Li, H.; Wang, X.; Tang, S.; Li, Q.; Wei, J.; Su, J. Evaluation of absorbable hemostatic agents of polyelectrolyte complexes using carboxymethyl starch and chitosan oligosaccharide both in vitro and in vivo. Biomater. Sci. 2018, 6, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte complexes of natural polymers and their biomedical applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Jasleen, K.; Gurpreet, K. Optimization of pH conditions and characterization of polyelectrolyte complexes between gellan gum and cationic guar gum. Polym. Adv. Technol. 2018, 29, 3035–3048. [Google Scholar]
- Ranganathan, P.; Mutharani, B.; Chen, S.M.; Sireesha, P. Biocompatible chitosan-pectin polyelectrolyte complex for simultaneous electrochemical determination of metronidazole and metribuzin. Carbohydr. Polym. 2019, 214, 317–327. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchis, R.; Alexandrescu, E.; Mihăescu, C.I.; Burlacu, S.; Trică, B.; Nistor, C.L.; Preda, S.; Scomoroscenco, C.; Gîfu, C.; et al. Adjusting some properties of poly(methacrylic acid) (nano)composite hydrogels by means of silicon-containing inorganic fillers. Int. J. Mol. Sci. 2022, 23, 10320. [Google Scholar] [CrossRef]
- Rashid, A.; Muhammad, T.; Imran, A.; Rehana, A.; Noorunnisa, P.; Robin, A.; Anwarul, H. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar]
- Aipeng, D.; Yang, Y.; Shimei, D. Tissue engineering 3D porous scaffolds prepared from electrospun recombinant human collagen (RHC) polypeptides/chitosan nanofibers. Appl. Sci. 2021, 11, 5096. [Google Scholar]
- Li, B.; Wang, J.; Gui, Q.; Yang, H. Drug-loaded chitosan film prepared via facile solution casting and air-drying of plain water-based chitosan solution for ocular drug delivery. Bioact. Mater. 2020, 5, 577–583. [Google Scholar] [CrossRef]
- Reay, S.L.; Jackson, E.L.; Ferreira, A.M.; Hilkens, C.M.U.; Novakovic, K. In vitro evaluation of the biodegradability of chitosan–genipin hydrogels. Mater. Adv. 2022, 3, 7946–7959. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Faezeh, N.; Raheleh, H.; Ali, S.; Marzieh, G. A new nanocomposite scaffold based on polyurethane and clay nanoplates for osteogenic differentiation of human mesenchymal stem cells in vitro. Mater. Sci. Eng. C 2019, 103, 109857. [Google Scholar]
- Ferreira, D.C.M.; Ferreira, S.O.; Alvarenga, E.S.; Soares, N.F.F.; Coimbra, J.S.R.; Oliveira, E.B. Polyelectrolyte complexes (PECs) obtained from chitosan and carboxymethylcellulose: A physicochemical and microstructural study. Carbohydr. Polym. Technol. Appl. 2022, 3, 100197. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. J. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Sivashankari, P.R.; Moorthi, A.; Abudhahir, K.M.; Prabaharan, M. Preparation and characterization of three-dimensional scaffolds based on hydroxypropyl chitosan-graft-graphene oxide. Int. J. Biol. Macromol. 2018, 110, 522–530. [Google Scholar] [CrossRef]
- Glen, H.K.; Daniel, J.H.; Qi, L.; Jennifer, A.L. Poly(acrylicacid)–poly(ethylene oxide) comb polymer effects on BaTiO3 nanoparticle suspension stability. J. Am. Ceram. Soc. 2004, 87, 181–186. [Google Scholar]
- Sathya Seeli, D.; Prabaharan, M. Guar gum oleate-graft-poly(methacrylic acid) hydrogel as a colon-specific controlled drug delivery carrier. Carbohydr. Polym. 2017, 158, 51–57. [Google Scholar] [CrossRef]
- Norcino, L.B.; de Oliveira, J.E.; Moreira, F.K.V.; Marconcini, J.M.; Mattoso, L.H.C. Rheological and thermo-mechanical evaluation of bio-based chitosan/pectin blends with tunable ionic cross-linking. Int. J. Biol. Macromol. 2018, 118B, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Xun, S.; Ying, X.; Qixin, Z.; Dongfeng, W. Novel antimicrobial and antioxidant chitosan derivatives prepared by green grafting with phenyllactic acid. Food Biophys. 2017, 12, 470–478. [Google Scholar]
- Han, G.; Huan, S.; Han, J.; Zhang, Z.; Wu, Q. Effect of acid hydrolysis conditions on the properties of cellulose nanoparticle reinforced polymethylmethacrylate composites. Materials 2014, 7, 16–29. [Google Scholar] [CrossRef]
- Wahid, F.; Duan, Y.X.; Hu, X.H.; Chu, L.Q.; Jia, S.R.; Cui, J.D.; Zhong, C. A facile construction of bacterial cellulose/ZnO nanocomposite films and their photocatalytic and antibacterial properties. Int. J. Biol. Macromol. 2019, 132, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Wu, E.C.; Zhang, S.; Hauser, C.A.E. Self-assembling peptides as cell-interactive scaffolds. Adv. Funct. Mater. 2012, 22, 456–468. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan, hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef]
- Cheng, T.N.; Liang, Q.Y.; Manoor, P.H.; Ong, C.N.; Yu, L.E.; Bay, B.H.; Baeg, G.H. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int. J. Nanomed. 2017, 12, 1621–1637. [Google Scholar]










| Type of PECs | Chitosan | PMA | Zn(CH3COO)2·2H2O |
|---|---|---|---|
| (Wt. %) | (Wt. %) | (mmol) | |
| CPMA | 2 | 2 | 0 |
| CPMAZnO-1 | 2 | 2 | 50 |
| CPMAZnO-2 | 2 | 2 | 75 |
| CPMAZnO-3 | 2 | 2 | 100 |
| CPMAZnO-4 | 2 | 2 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathya Seeli, D.; Das, A.; Prabaharan, M. Zinc Oxide–Incorporated Chitosan–Poly(methacrylic Acid) Polyelectrolyte Complex as a Wound Healing Material. J. Funct. Biomater. 2023, 14, 228. https://doi.org/10.3390/jfb14040228
Sathya Seeli D, Das A, Prabaharan M. Zinc Oxide–Incorporated Chitosan–Poly(methacrylic Acid) Polyelectrolyte Complex as a Wound Healing Material. Journal of Functional Biomaterials. 2023; 14(4):228. https://doi.org/10.3390/jfb14040228
Chicago/Turabian StyleSathya Seeli, David, Abinash Das, and Mani Prabaharan. 2023. "Zinc Oxide–Incorporated Chitosan–Poly(methacrylic Acid) Polyelectrolyte Complex as a Wound Healing Material" Journal of Functional Biomaterials 14, no. 4: 228. https://doi.org/10.3390/jfb14040228
APA StyleSathya Seeli, D., Das, A., & Prabaharan, M. (2023). Zinc Oxide–Incorporated Chitosan–Poly(methacrylic Acid) Polyelectrolyte Complex as a Wound Healing Material. Journal of Functional Biomaterials, 14(4), 228. https://doi.org/10.3390/jfb14040228