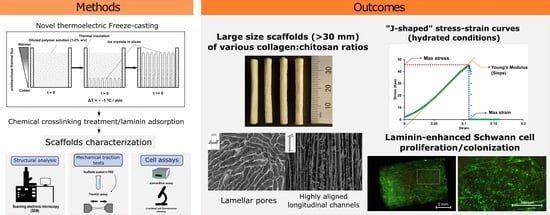
Thermoelectric Freeze-Casting of Biopolymer Blends: Fabrication and Characterization of Large-Size Scaffolds for Nerve Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biopolymers Preparation
2.3. Scaffold Preparation for Thermoelectric-Based Unidirectional Freeze-Casting
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Scaffold Pore Analysis
2.6. Evaluation of Microstructural Feature Alignment
2.7. Mechanical Characterization
2.8. Cell Culture
2.9. Seeding of the Scaffolds
2.10. Cell Viability Tests and Colonization
2.11. Immunostainings
2.12. Statistical Methods
3. Results
3.1. Pore Microstructure Characterization
3.2. Microstructural Features’ Organization and Alignment
3.3. Mechanical Properties
3.4. Quantitative Analyses of Scaffold Cytocompatibility
3.5. Cell Colonization and Viability
3.6. Immunostaining
4. Discussion
4.1. Scaffold Structure Obtained from Thermoelectric Freeze-Casting
4.2. Mechanical Properties under Traction
4.3. Schwann Cell Colonization, Survival, Proliferation, and Functionality at the Contact of the Scaffolds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bailey, R.; Kaskutas, V.; Fox, I.; Baum, C.M.; Mackinnon, S.E. Effect of Upper Extremity Nerve Damage on Activity Participation, Pain, Depression, and Quality of Life. J. Hand Surg. Am. 2009, 34, 1682–1688. [Google Scholar] [CrossRef]
- Siemionow, M.; Brzezicki, G. Chapter 8 Current Techniques and Concepts in Peripheral Nerve Repair, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 87. [Google Scholar]
- Noble, J.; Munro, C.A.; Prasad, V.S.S.V.; Midha, R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma Inj. Infect. Crit. Care 1998, 45, 116–122. [Google Scholar] [CrossRef]
- López-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Silva, T.H.; Oliveira, J.M. Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater. Sci. Eng. 2017, 3, 3098–3122. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, J.A. Peripheral nerve injuries: A retrospective survey of 456 cases. Muscle Nerve 2006, 34, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Braza, D.; Rice, J.B.; Dillingham, T. The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil. 2008, 87, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Eslami, V.; Rahimi-Movaghar, V. The incidence of peripheral nerve injury in trauma patients in Iran. Ulus. Travma ve Acil Cerrahi Derg. 2011, 17, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [Green Version]
- Oberlin, C.; Rantissi, M. Gunshot injuries to the nerves. Chir. Main 2011, 30, 176–182. [Google Scholar] [CrossRef]
- Birch, R.; Misra, P.; Stewart, M.P.M.; Eardley, W.P.G.; Ramasamy, A.; Brown, K.; Shenoy, R.; Anand, P.; Clasper, J.; Dunn, R.; et al. Nerve injuries sustained during warfare: Part I—Epidemiology. J. Bone Joint Surg. Br. 2012, 94, 523–528. [Google Scholar] [CrossRef]
- Birch, R.; Misra, P.; Stewart, M.P.M.; Eardley, W.P.G.; Ramasamy, A.; Brown, K.; Shenoy, R.; Anand, P.; Clasper, J.; Dunn, R.; et al. Nerve injuries sustained during warfare: Part II: Outcomes. J. Bone Joint Surg. Br. 2012, 94, 529–535. [Google Scholar] [CrossRef]
- Mitchell, S.W.; Morehouse, G.R.; Keen, W.W. THE CLASSIC: Gunshot Wounds and Other Injuries of Nerves. Clin. Orthop. Relat. Res. 2007, 458, 35–39. [Google Scholar] [CrossRef]
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences; Taylor & Francis: New York, NY, USA, 1988; Volume 44. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Belmont, P.J.; Schoenfeld, A.J.; Goodman, G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: Orthopaedic burden of disease. J. Surg. Orthop. Adv. 2010, 19, 2–7. [Google Scholar]
- Birch, R.; Raji, A.R.M. Repair of median and ulnar nerves. Primary suture is best. J. Bone Jt. Surg. Ser. B 1991, 73, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Rydevik, B. Effects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineurium. J. Bone Joint Surg. Br. 1973, 55, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Millesi, H.; Meissl, G.; Berger, A. The interfascicular nerve-grafting of the median and ulnar nerves. J. Bone Joint Surg. Am. 1972, 54, 727–750. [Google Scholar] [CrossRef]
- de Medinaceli, L.; Prayon, M.; Merle, M. Percentage of nerve injuries in which primary repair can be achieved by end-to-end approximation: Review of 2,181 nerve lesions. Microsurgery 1993, 14, 244–246. [Google Scholar] [CrossRef]
- Weber, R.A.; Breidenbach, W.C.; Brown, R.E.; Jabaley, M.E.; Mass, D.P. A Randomized Prospective Study of Polyglycolic Acid Conduits for Digital Nerve Reconstruction in Humans. Plast. Reconstr. Surg. 2000, 106, 1036–1045. [Google Scholar] [CrossRef]
- Lohmeyer, J.A.; Sommer, B.; Siemers, F.; Mailänder, P. Nerve injuries of the upper extremity-expected outcome and clinical examination. Plast. Surg. Nurs. 2009, 29, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, S.; Facca, S.; Diaconu, M.; Gouzou, S.; Liverneaux, P. Experience of using the bioresorbable copolyester poly(DL-lactide-ε-caprolactone) nerve conduit guide NeurolacTM for nerve repair in peripheral nerve defects: Report on a series of 28 lesions. J. Hand Surg. 2012, 37, 342–349. [Google Scholar] [CrossRef]
- Mauch, J.T.; Bae, A.; Shubinets, V.; Lin, I.C. A Systematic Review of Sensory Outcomes of Digital Nerve Gap Reconstruction With Autograft, Allograft, and Conduit. Ann. Plast. Surg. 2019, 82 (Suppl. 3), S247–S255. [Google Scholar] [CrossRef]
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Zhang, P.; Xiu, J.; Yang, Y.; Zhang, F.; et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757. [Google Scholar] [CrossRef]
- Kerns, J.; Piponov, H.; Helder, C.; Amirouche, F.; Solitro, G.; Gonzalez, M. Mechanical Properties of the Human Tibial and Peroneal Nerves Following Stretch With Histological Correlations. Anat. Rec. 2019, 302, 2030–2039. [Google Scholar] [CrossRef]
- Muangsanit, P.; Day, A.; Dimiou, S.; Ataç, A.F.; Kayal, C.; Park, H.; Nazhat, S.N.; Phillips, J.B. Rapidly formed stable and aligned dense collagen gels seeded with Schwann cells support peripheral nerve regeneration. J. Neural Eng. 2020, 17, 046036. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, H.M.; Mishra, P.; Kohn, J. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J. Mater. Sci. Mater. Med. 2015, 26, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Ruiter, G.C.W.; Wang, H.; Knight, A.M.; Spinner, R.J.; Yaszemski, M.J.; Windebank, A.J.; Pandit, A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 2010, 31, 5789–5797. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Billiar, K.L.; Windebank, A.J.; Pandit, A. Multichanneled Collagen Conduits for Peripheral Nerve Regeneration: Design, Fabrication, and Characterization. Tissue Eng. Part C Methods 2010, 16, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res. Int. 2016, 2016, 3856262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Qu, W.; Li, D.; Shi, K.; Li, R.; Han, Y.; Jin, E.; Ding, J.; Chen, X. Functional Polymer-Based Nerve Guide Conduits to Promote Peripheral Nerve Regeneration. Adv. Mater. Interfaces 2020, 7, 2000225. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Schecter, M.; Donius, A.E.; Hunger, P.M. Biomaterials by freeze casting. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2099–2121. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef]
- Christiansen, C.D.; Nielsen, K.K.; Bjørk, R. Novel freeze-casting device with high precision thermoelectric temperature control for dynamic freezing conditions. Rev. Sci. Instrum. 2020, 91, 033904. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Christiansen, C.D.; Engelbrecht, K.; Nielsen, K.K.; Bjørk, R.; Bahl, C.R.H. Characterization of Freeze-Cast Micro-Channel Monoliths as Active and Passive Regenerators. Front. Energy Res. 2020, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Riyad, M.F.; Mahmoudi, M.; Minary-Jolandan, M. Manufacturing and Thermal Shock Characterization of Porous Yttria Stabilized Zirconia for Hydrogen Energy Systems. Ceramics 2022, 5, 472–483. [Google Scholar] [CrossRef]
- Doman, E.A. Modelling The Mechanical Properties of Peripheral Nerves and Experimental Tissue-Engineered Conduit Designs; University College London: London, UK, 2021. [Google Scholar]
- Fregnan, F.; Muratori, L.; Bassani, G.A.; Crosio, A.; Biagiotti, M.; Vincoli, V.; Carta, G.; Pierimarchi, P.; Geuna, S.; Alessandrino, A.; et al. Preclinical Validation of SilkBridgeTM for Peripheral Nerve Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 835. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zheng, Z.; Yan, J.; Zhang, L.; Li, Y.; Zhang, J.; Li, G.; Wang, X.; Kaplan, D. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair. Acta Biomater. 2021, 134, 116–130. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Lawal, G.I.; Gbenebor, O.P. Characteristics of Biodegradable Implants. J. Miner. Mater. Charact. Eng. 2014, 2, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Suchý, T.; Šupová, M.; Bartoš, M.; Sedláček, R.; Piola, M.; Soncini, M.; Fiore, G.B.; Sauerová, P.; Kalbáčová, M.H. Dry versus hydrated collagen scaffolds: Are dry states representative of hydrated states? J. Mater. Sci. Mater. Med. 2018, 29, 20. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.; Blanco, M.D.; Davidenko, N.; Cameron, R.E. Tailoring chitosan/collagen scaffolds for tissue engineering: Effect of composition and different crosslinking agents on scaffold properties. Carbohydr. Polym. 2015, 132, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and recent developments of chitosan in peripheral nerve surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, C.R.; López-Cebral, R.; Silva-Correia, J.; Silva, J.M.; Mano, J.F.; Silva, T.H.; Freier, T.; Reis, R.L.; Oliveira, J.M. Investigation of cell adhesion in chitosan membranes for peripheral nerve regeneration. Mater. Sci. Eng. C 2017, 71, 1122–1134. [Google Scholar] [CrossRef] [Green Version]
- Dobrzański, L.A.; Hudecki, A.; Chladek, G.; Król, W.; Mertas, A. Biodegradable and antimicrobial polycaprolactone nanofibers with and without silver precipitates. Arch. Mater. Sci. Eng. 2015, 76, 5–26. [Google Scholar]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Kennedy, C.J.; Wess, T.J. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Belfiore, L.; Chu, T.-H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair From Studies of Development and Injury. Front. Mol. Neurosci. 2021, 13, 608442. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.M.A.P.; Day, A.G.E.; Redl, H.; Phillips, J. An Optimized Collagen-Fibrin Blend Engineered Neural Tissue Promotes Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Boecker, A.; Tank, J.; Altinova, H.; Deumens, R.; Dabhi, C.; Tolba, R.; Weis, J.; Brook, G.A.; Pallua, H.; et al. Efficient bridging of 20 mm rat sciatic nerve lesions with a longitudinally micro-structured collagen scaffold. Biomaterials 2016, 75, 112–122. [Google Scholar] [CrossRef]
- Chen, Z.-L.L.; Strickland, S. Laminin γ1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 2003, 163, 889–899. [Google Scholar] [CrossRef] [Green Version]
- McKee, K.K.; Yang, D.-H.; Patel, R.; Chen, Z.-L.; Strickland, S.; Takagi, J.; Sekiguchi, K.; Yurchenko, P.D. Schwann cell myelination requires integration of laminin activities. J. Cell Sci. 2012, 125, 4609–4619. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Luo, Z.J.; Zheng, Y.; Hu, X.Y.; Ye, Z.X. Rapid repair and regeneration of damaged rabbit sciatic nerves by tissue-engineered scaffold made from nano-silver and collagen type I. Injury 2010, 41, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Pot, M.W.; Faraj, K.A.; Adawy, A.; Enckevort, W.J.P.; van Moerkerk, H.T.B.; Vlieg, E.; Daamen, W.F.; van Kuppevelt, T.H. Versatile wedge-based system for the construction of unidirectional collagen scaffolds by directional freezing: Practical and theoretical considerations. ACS Appl. Mater. Interfaces 2015, 7, 8495–8505. [Google Scholar] [CrossRef] [PubMed]
- Divakar, P.; Yin, K.; Wegst, U.G.K. Anisotropic freeze-cast collagen scaffolds for tissue regeneration: How processing conditions affect structure and properties in the dry and fully hydrated states. J. Mech. Behav. Biomed. Mater. 2018, 90, 350–364. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, L.; Shi, X.; Xia, B.; Liu, Z.; Zhu, S.; Yang, Y.; Ma, T.; Cheng, P.; Luo, K.; et al. A compound scaffold with uniform longitudinally oriented guidance cues and a porous sheath promotes peripheral nerve regeneration in vivo. Acta Biomater. 2018, 68, 223–236. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Bradski, G. The OpenCV Library. Dr. Dobb’s J. Softw. Tools 2000, 120, 122–125. [Google Scholar]
- He, L.; Chao, Y.; Suzuki, K. A New Two-Scan Algorithm for Labeling Connected Components in Binary Images. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012; Volume II. [Google Scholar]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; van de Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Parent, G.; Huppé, N.; Langelier, E. Low Stress Tendon Fatigue is a Relatively Rapid Process in the Context of Overuse Injuries. Ann. Biomed. Eng. 2011, 39, 1535–1545. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Wangensteen, K.J.; Kalliainen, L.K. Collagen Tube Conduits in Peripheral Nerve Repair: A Retrospective Analysis. HAND 2010, 5, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Krych, A.J.; Rooney, G.E.; Chen, B.; Schermerhorn, T.C.; Ameenuddin, S.; Gross, L.; Moore, M.J.; Currier, B.L.; Spinner, R.J.; Friedman, J.A.; et al. Relationship between scaffold channel diameter and number of regenerating axons in the transected rat spinal cord. Acta Biomater. 2009, 5, 2551–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, K.; Mueller, R.; Caioni, M.; Prang, P.; Bogdahn, U.; Kunz, W.; Weidner, N. Increasing capillary diameter and the incorporation of gelatin enhance axon outgrowth in alginate-based anisotropic hydrogels. Acta Biomater. 2011, 7, 2826–2834. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Yoon, C.; Giger, R.J.; Sakamoto, J. Engineering a platform for nerve regeneration with direct application to nerve repair technology. Biomaterials 2019, 216, 119263. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, K.-Y.; Chen, Y.-F.; Li, X.-J.; Zhu, S.; Zhang, Z.-Y.; Wang, X.; Duan, L.-N.; Luo, Z.-J.; Du, J.-J.; et al. Beagle sciatic nerve regeneration across a 30 mm defect bridged by chitosan/PGA artificial nerve grafts. Injury 2018, 49, 1477–1484. [Google Scholar] [CrossRef]
- Rieu, C.; Parisi, C.; Mosser, G.; Haye, B.; Coradin, T.; Fernandes, F.M.; Trichet, L. Topotactic Fibrillogenesis of Freeze-Cast Microridged Collagen Scaffolds for 3D Cell Culture. ACS Appl. Mater. Interfaces 2019, 11, 14672–14683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunger, P.M.; Donius, A.E.; Wegst, U.G.K. Structure–property-processing correlations in freeze-cast composite scaffolds. Acta Biomater. 2013, 9, 6338–6348. [Google Scholar] [CrossRef]
- Hibbitts, A.J.; Kočí, Z.; Kneafsey, S.; Matsiko, A.; Žilić, L.; Dervan, A.; Hinton, P.; Chen, G.; Cavanagh, B.; Dowling, J.K.; et al. Multi-factorial nerve guidance conduit engineering improves outcomes in inflammation, angiogenesis and large defect nerve repair. Matrix Biol. 2022, 106, 34–57. [Google Scholar] [CrossRef]
- Song, S.; Wang, X.; Wang, T.; Yu, Q.; Hou, Z.; Zhu, Z.; Li, R. Additive Manufacturing of Nerve Guidance Conduits for Regeneration of Injured Peripheral Nerves. Front. Bioeng. Biotechnol. 2020, 8, 590596. [Google Scholar] [CrossRef]
- Yin, K.; Divakar, P.; Wegst, U.G.K. Structure-property-processing correlations of longitudinal freeze-cast chitosan scaffolds for biomedical applications. J. Mech. Behav. Biomed. Mater. 2021, 121, 104589. [Google Scholar] [CrossRef]
- Clements, B.A.; Bushman, J.; Murthy, N.S.; Ezra, M.; Pastore, C.M.; Kohn, J. Design of barrier coatings on kink-resistant peripheral nerve conduits. J. Tissue Eng. 2016, 7, 2041731416629471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Versteegden, L.R.; Hoogenkamp, H.R.; Lomme, R.M.; van Goor, H.; Tiemessen, D.M.; Geutjes, P.J.; Oosterwijk, E.; Feitz, W.F.; Hafmans, T.G.; Verdonschot, N.; et al. Design of an elasticized collagen scaffold: A method to induce elasticity in a rigid protein. Acta Biomater. 2016, 44, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Meng, H.; Chang, B.; Hong, L.; Li, R.; Liu, G.; Cheng, X.; Tang, H.; Liu, P.; Sun, Y.; et al. Novel 3-D helix-flexible nerve guide conduits repair nerve defects. Biomaterials 2019, 207, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Champagne, N.; Cousineau-Pelletier, P.; Parent, G.; Langelier, E. Preparation of rat tail tendons for biomechanical and mechanobiological studies. J. Vis. Exp. 2010, 41, 3–7. [Google Scholar]
- Ma, Y.; Feng, X.; Rogers, J.A.; Huang, Y.; Zhang, Y. Design and application of ‘J-shaped’ stress-strain behavior in stretchable electronics: A review. Lab Chip 2017, 17, 1689–1704. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Schaible, E.; Stewart, P.; Ritchie, R.O.; Meyers, M.A. On the tear resistance of skin. Nat. Commun. 2015, 6, 6649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taravel, M.N.; Domard, A. Relation between the physicochemical characteristics of collagen and its interactions with chitosan: I. Biomaterials 1993, 14, 930–938. [Google Scholar] [CrossRef]
- Taravel, M.N.; Domard, A. Collagen and its interaction with chitosan. Biomaterials 1995, 16, 865–871. [Google Scholar] [CrossRef]
- Taravel, M. Collagen and its interactions with chitosan III. Some biological and mechanical properties. Biomaterials 1996, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.E.; Born, W. Stimulation of neurite outgrowth in a human nerve scaffold designed for peripheral nerve reconstruction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 194–202. [Google Scholar] [CrossRef]
- Belin, S.; Zuloaga, K.L.; Poitelon, Y. Influence of mechanical stimuli on schwann cell biology. Front. Cell. Neurosci. 2017, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, D.; Wang, Y.; Sun, B.; Li, D.; Morsi, Y.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Laminin-coated nerve guidance conduits based on poly(l-lactide-: Co-glycolide) fibers and yarns for promoting Schwann cells’ proliferation and migration. J. Mater. Chem. B 2017, 5, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, T.; Perez-Piñera, P.; Díaz-Esnal, B.; Vega, J.A. S-100 proteins in the human peripheral nervous system. Microsc. Res. Tech. 2003, 60, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. Signals that determine Schwann cell identity. J. Anat. 2002, 200, 367–376. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hoshikawa, S.; Ueno, T.; Hirata, M.; Saito, T.; Ikeda, T.; Kawaguchi, H.; Nakamura, K.; Tanaka, S.; Ogata, T. SOX10 Transactivates S100B to Suppress Schwann Cell Proliferation and to Promote Myelination. PLoS ONE 2014, 9, e115400. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Chen, Y.; Dai, F.; Yu, X. Chitosan-based nerve guidance conduit with microchannels and nanofibers promotes schwann cells migration and neurite growth. Colloids Surf. B Biointerfaces 2023, 221, 112929. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Liu, C.; Kray, J.; Chan, C. Schwann Cells Enhance Penetration of Regenerated Axons into Three-Dimensional Microchannels. Tissue Eng. Regen. Med. 2018, 15, 351–361. [Google Scholar] [CrossRef]
- Mobini, S.; Kuliasha, C.A.; Siders, Z.A.; Bohmann, N.A.; Jamal, S.-M.; Judy, J.W.; Schmidt, C.E.; Brennan, A.B. Microtopographical patterns promote different responses in fibroblasts and Schwann cells: A possible feature for neural implants. J. Biomed. Mater. Res. Part A 2021, 109, 64–76. [Google Scholar] [CrossRef]
- Zennifer, A.; Thangadurai, M.; Sundaramurthi, D.; Sethuraman, S. Additive manufacturing of peripheral nerve conduits—Fabrication methods, design considerations and clinical challenges. SLAS Technol. 2023, 28, 102–126. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Ehsani, A.; Khademi, A.; Asefnejad, A.; Shahriari, S.; Soleimani, M.; Nejad, M.G.; Saber-Samandari, S.; Khandan, A. Application of 3D Bioprinters for Dental Pulp Regeneration and Tissue Engineering (Porous architecture). Transp. Porous Media 2022, 142, 265–293. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, D.N.; Pinto, G.B.A.; Cartarozzi, L.P.; Rodrigues de Olivera, A.L.; Bovolato, A.L.C.; de Carvalho, M.; da Silva, J.V.L.; Dernowsek, J.A.; Golim, M.; Barraviera, B.; et al. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res. Ther. 2021, 12, 303. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monfette, V.; Choinière, W.; Godbout-Lavoie, C.; Pelletier, S.; Langelier, È.; Lauzon, M.-A. Thermoelectric Freeze-Casting of Biopolymer Blends: Fabrication and Characterization of Large-Size Scaffolds for Nerve Tissue Engineering Applications. J. Funct. Biomater. 2023, 14, 330. https://doi.org/10.3390/jfb14060330
Monfette V, Choinière W, Godbout-Lavoie C, Pelletier S, Langelier È, Lauzon M-A. Thermoelectric Freeze-Casting of Biopolymer Blends: Fabrication and Characterization of Large-Size Scaffolds for Nerve Tissue Engineering Applications. Journal of Functional Biomaterials. 2023; 14(6):330. https://doi.org/10.3390/jfb14060330
Chicago/Turabian StyleMonfette, Vincent, William Choinière, Catherine Godbout-Lavoie, Samuel Pelletier, Ève Langelier, and Marc-Antoine Lauzon. 2023. "Thermoelectric Freeze-Casting of Biopolymer Blends: Fabrication and Characterization of Large-Size Scaffolds for Nerve Tissue Engineering Applications" Journal of Functional Biomaterials 14, no. 6: 330. https://doi.org/10.3390/jfb14060330
APA StyleMonfette, V., Choinière, W., Godbout-Lavoie, C., Pelletier, S., Langelier, È., & Lauzon, M. -A. (2023). Thermoelectric Freeze-Casting of Biopolymer Blends: Fabrication and Characterization of Large-Size Scaffolds for Nerve Tissue Engineering Applications. Journal of Functional Biomaterials, 14(6), 330. https://doi.org/10.3390/jfb14060330