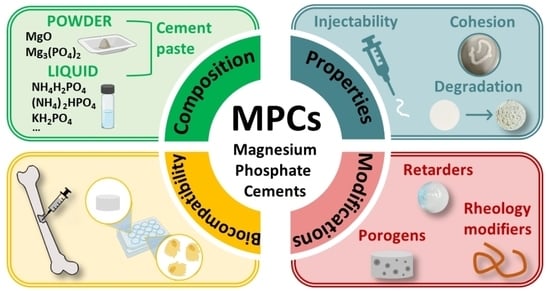
An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials
Abstract
:1. Introduction
2. Bone Tissue and Repair Strategies
3. Bone Cements
4. Magnesium Phosphate Cements: Preparation and Properties
4.1. Preparation of MPCs
4.2. Properties of MPCs
5. Modifications of MPCs
5.1. Retarders
5.2. Biorelevant Molecules
5.3. Polymers
6. Biological Performance
6.1. In Vitro Experiments
6.2. In Vivo Experiments
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salari, N.; Darvishi, N.; Bartina, Y.; Larti, M.; Kiaei, A.; Hemmati, M.; Shohaimi, S.; Mohammadi, M. Global Prevalence of Osteoporosis among the World Older Adults: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Metabolic Bone Disease—Chapter 101. In Kelley and Firestein’s Textbook of Rheumatology, 10th ed.; Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes, I.B., O’Dell, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1730–1750.e4. ISBN 978-0-323-31696-5. [Google Scholar]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic Burden of Osteoporosis in the World: A Systematic Review. Med. J. Islam. Repub. Iran 2020, 34, 154. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.-M. A Review of Calcium Phosphate Cements and Acrylic Bone Cements as Injectable Materials for Bone Repair and Implant Fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Healthc. Mater. 2020, 9, 2000349. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium Phosphate Cements for Bone Substitution: Chemistry, Handling and Mechanical Properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Self-Setting Calcium Orthophosphate Formulations: Cements, Concretes, Pastes and Putties. Int. J. Mater. Chem. 2012, 1, 1–48. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium Phosphate Cements: Optimization toward Biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.-P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical Review: Injectability of Calcium Phosphate Pastes and Cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors Influencing the Drug Release from Calcium Phosphate Cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone Cements for Percutaneous Vertebroplasty and Balloon Kyphoplasty: Current Status and Future Developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium Phosphate Cement Systems for Hard Tissue Applications: A Review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Seehra, S.S.; Gupta, S.; Kumar, S. Rapid Setting Magnesium Phosphate Cement for Quick Repair of Concrete Pavements—Characterisation and Durability Aspects. Cem. Concr. Res. 1993, 23, 254–266. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-Based Bioceramics in Orthopedic Applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Qi, C.; Cai, K. Biodegradable Magnesium Phosphates in Biomedical Applications. J. Mater. Chem. B 2022, 10, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Bavya Devi, K.; Lalzawmliana, V.; Saidivya, M.; Kumar, V.; Roy, M.; Kumar Nandi, S. Magnesium Phosphate Bioceramics for Bone Tissue Engineering. Chem. Rec. 2022, 22, e202200136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B. In Vitro and in Vivo Research Advancements on the Magnesium Phosphate Cement Biomaterials: A Review. Materialia 2020, 13, 100852. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical Properties and the Hierarchical Structure of Bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Hardy, E.; Fernandez-Patron, C. Destroy to Rebuild: The Connection Between Bone Tissue Remodeling and Matrix Metalloproteinases. Front. Physiol. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.M. An Overview of Bone Cells and Their Regulating Factors of Differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Holmes, D. Closing the Gap. Nature 2017, 550, S194–S195. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [Green Version]
- Ginebra, M.-P.; Montufar, E.B. Cements as Bone Repair Materials. In Bone Repair Biomaterials, 2nd ed.; Pawelec, K.M., Planell, J.A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 233–271. ISBN 978-0-08-102451-5. [Google Scholar]
- Liu, D.; Cui, C.; Chen, W.; Shi, J.; Li, B.; Chen, S. Biodegradable Cements for Bone Regeneration. J. Funct. Biomater. 2023, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Chan, E.K.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate Bone Cements and Additives: A Review of the Literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Liu, X.; Lian, X.; Guo, Z.; Jiang, H.-J.; Cui, F.-Z. Improved Workability of Injectable Calcium Sulfate Bone Cement by Regulation of Self-Setting Properties. Mater. Sci. Eng. C 2013, 33, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, L.; Yang, X.; Wang, F.; Feng, J.; Hua, K.; Li, Q.; Hu, Y. Demineralized Bone Matrix Carriers and Their Clinical Applications: An Overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.; Chow, L. A New Calcium Phosphate Water Setting Cement. In Cements Research Progress; Brown, W.E., Ed.; American Ceramic Society: Westerville, OH, USA, 1986; pp. 352–379. [Google Scholar]
- Zwingenberger, S.; Nich, C.; Valladares, R.D.; Yao, Z.; Stiehler, M.; Goodman, S.B. Recommendations and Considerations for the Use of Biologics in Orthopedic Surgery. BioDrugs 2012, 26, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.E.; Grover, L.M.; Gbureck, U. Ionic Modification of Calcium Phosphate Cement Viscosity. Part II: Hypodermic Injection and Strength Improvement of Brushite Cement. Biomaterials 2004, 25, 2197–2203. [Google Scholar] [CrossRef]
- Tronco, M.C.; Cassel, J.B.; dos Santos, L.A. α-TCP-Based Calcium Phosphate Cements: A Critical Review. Acta Biomater. 2022, 151, 70–87. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and Mechanical Performance and Degradation Characteristics of Calcium Phosphate Cements in Large Animals and Humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Sugawara, A.; Asaoka, K.; Ding, S.-J. Calcium phosphate-based cements: Clinical needs and recent progress. J. Mater. Chem. B 2013, 1, 1081–1089. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium Phosphate Cements for Bone Engineering and Their Biological Properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [Green Version]
- Hughes, E.; Yanni, T.; Jamshidi, P.; Grover, L.M. Inorganic Cements for Biomedical Application: Calcium Phosphate, Calcium Sulphate and Calcium Silicate. Adv. Appl. Ceram. 2015, 114, 65–76. [Google Scholar] [CrossRef]
- Bohner, M. Design of Ceramic-Based Cements and Putties for Bone Graft Substitution. Eur. Cells Mater. 2010, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kanter, B.; Geffers, M.; Ignatius, A.; Gbureck, U. Control of in Vivo Mineral Bone Cement Degradation. Acta Biomater. 2014, 10, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Theiss, F.; Apelt, D.; Hirsiger, W.; Houriet, R.; Rizzoli, G.; Gnos, E.; Frei, C.; Auer, J.A.; von Rechenberg, B. Compositional Changes of a Dicalcium Phosphate Dihydrate Cement after Implantation in Sheep. Biomaterials 2003, 24, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Driessens, F.C.M.; Boltong, M.G.; Zapatero, M.I.; Verbeeck, R.M.H.; Bonfield, W.; Bérmúdez, O.; Fernández, E.; Ginebra, M.P.; Planell, J.A. In Vivo Behaviour of Three Calcium Phosphate Cements and a Magnesium Phosphate Cement. J. Mater. Sci. Mater. Med. 1995, 6, 272–278. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [Green Version]
- Lothenbach, B.; Xu, B.; Winnefeld, F. Thermodynamic Data for Magnesium (Potassium) Phosphates. Appl. Geochem. 2019, 111, 104450. [Google Scholar] [CrossRef]
- Taylor, A.W.; Frazier, A.W.; Gurney, E.L.; Smith, J.P. Solubility Products of Di- and Trimagnesium Phosphates and the Dissociation of Magnesium Phosphate Solutions. Trans. Faraday Soc. 1963, 59, 1585–1589. [Google Scholar] [CrossRef]
- Taylor, A.W.; Frazier, A.W.; Gurney, E.L. Solubility Products of Magnesium Ammonium and Magnesium Potassium Phosphates. Trans. Faraday Soc. 1963, 59, 1580–1584. [Google Scholar] [CrossRef]
- Frazier, A.W.; Lehr, J.R.; Smith, J.P. The Magnesium Phosphates Hannayite, Schertelite and Bobierrite. Am. Mineral. 1963, 48, 635–641. [Google Scholar]
- Abdelrazig, B.E.I.; Sharp, J.H.; El-Jazairi, B. The Chemical Composition of Mortars Made from Magnesia-Phosphate Cement. Cem. Concr. Res. 1988, 18, 415–425. [Google Scholar] [CrossRef]
- Hall, D.A.; Stevens, R.; Jazairi, B.E. Effect of Water Content on the Structure and Mechanical Properties of Magnesia-Phosphate Cement Mortar. J. Am. Ceram. Soc. 1998, 81, 1550–1556. [Google Scholar] [CrossRef]
- Soudée, E.; Péra, J. Mechanism of Setting Reaction in Magnesia-Phosphate Cements. Cem. Concr. Res. 2000, 30, 315–321. [Google Scholar] [CrossRef]
- Mestres, G.; Ginebra, M.-P. Novel Magnesium Phosphate Cements with High Early Strength and Antibacterial Properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Mestres, G.; Aguilera, F.S.; Manzanares, N.; Sauro, S.; Osorio, R.; Toledano, M.; Ginebra, M.P. Magnesium Phosphate Cements for Endodontic Applications with Improved Long-Term Sealing Ability. Int. Endod. J. 2014, 47, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Lai, Z.; Lai, X.; Lu, Z.; Lv, S. Preparation and Characteristics of Magnesium Phosphate Cement Based Porous Materials. Constr. Build. Mater. 2016, 127, 712–723. [Google Scholar] [CrossRef]
- Liao, J.; Lu, S.; Duan, X.; Xie, Y.; Zhang, Y.; Li, Y.; Zhou, A. Affecting Mechanism of Chitosan on Water Resistance of Magnesium Phosphate Cement. Int. J. Appl. Ceram. Technol. 2018, 15, 514–521. [Google Scholar] [CrossRef]
- You, C.; Qian, J.; Qin, J.; Wang, H.; Wang, Q.; Ye, Z. Effect of Early Hydration Temperature on Hydration Product and Strength Development of Magnesium Phosphate Cement (MPC). Cem. Concr. Res. 2015, 78, 179–189. [Google Scholar] [CrossRef]
- Jun, L.; Yong-sheng, J.; Guodong, H.; Cheng, J. Retardation and Reaction Mechanisms of Magnesium Phosphate Cement Mixed with Glacial Acetic Acid. RSC Adv. 2017, 7, 46852–46857. [Google Scholar] [CrossRef] [Green Version]
- Mestres, G.; Abdolhosseini, M.; Bowles, W.; Huang, S.-H.; Aparicio, C.; Gorr, S.-U.; Ginebra, M.-P. Antimicrobial Properties and Dentin Bonding Strength of Magnesium Phosphate Cements. Acta Biomater. 2013, 9, 8384–8393. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.; Huang, G.; Xu, Z.; Yan, G. Properties and Reaction Mechanisms of Magnesium Phosphate Cement Mixed with Acetic Acid. KSCE J. Civ. Eng. 2018, 22, 231–235. [Google Scholar] [CrossRef]
- Lu, X.; Chen, B. Experimental Study of Magnesium Phosphate Cements Modified by Metakaolin. Constr. Build. Mater. 2016, 123, 719–726. [Google Scholar] [CrossRef]
- Qin, Z.; Ma, C.; Zheng, Z.; Long, G.; Chen, B. Effects of Metakaolin on Properties and Microstructure of Magnesium Phosphate Cement. Constr. Build. Mater. 2020, 234, 117353. [Google Scholar] [CrossRef]
- Ma, C.; Wang, F.; Zhou, H.; Jiang, Z.; Ren, W.; Du, Y. Effect of Early-Hydration Behavior on Rheological Properties of Borax-Admixed Magnesium Phosphate Cement. Constr. Build. Mater. 2021, 283, 122701. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; de Paula, G.R.; Morelli, M.R. Effect of MgO/NH4H2PO4 Ratio on the Properties of Magnesium Phosphate Cements. Mat. Res. 2020, 23, e20200034. [Google Scholar] [CrossRef]
- Babaie, E.; Lin, B.; Bhaduri, S.B. A New Method to Produce Macroporous Mg-Phosphate Bone Growth Substitutes. Mater. Sci. Eng. C 2017, 75, 602–609. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Agnelli, J.A.M.; Morelli, M.R. Study of Mechanical Properties and Durability of Magnesium Phosphate Cement Matrix Containing Grinding Dust. Mater. Res. 2013, 16, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.A.; Stevens, R.; El-Jazairi, B. The Effect of Retarders on the Microstructure and Mechanical Properties of Magnesia–Phosphate Cement Mortar. Cem. Concr. Res. 2001, 31, 455–465. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, B.; Ahmad, M.R. Effects of Alumina as an Effective Constituent of Metakaolin on Properties of Magnesium Phosphate Cements. J. Mater. Civ. Eng. 2019, 31, 04019147. [Google Scholar] [CrossRef]
- Zhenyu, L.; Yang, H.; Xiaojie, F.; Zhongyuan, L.; Shuzhen, L. Preparation of Porous Materials by Magnesium Phosphate Cement with High Permeability. Adv. Mater. Sci. Eng. 2018, 2018, e5910560. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, D.V.; Paula, G.R.; Morelli, M.R. Effect of Boric Acid Content on the Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2019, 214, 557–564. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Dong, B.; Wang, Y.; Xing, F. Influence Mechanisms of Fly Ash in Magnesium Ammonium Phosphate Cement. Constr. Build. Mater. 2022, 314, 125581. [Google Scholar] [CrossRef]
- Gharsallah, S.; Alsawi, A.; Hammami, B.; Khitouni, M.; Charnay, C.; Chemingui, M. Synthesis and Characterization of New Composite Materials Based on Magnesium Phosphate Cement for Fluoride Retention. Materials 2023, 16, 718. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, Y.; Zhao, Z.; Paxton, J.Z.; Grover, L.M. Synthesis and in Vitro Degradation of a Novel Magnesium Oxychloride Cement. J. Biomed. Mater. Res. Part A 2015, 103, 194–202. [Google Scholar] [CrossRef]
- Fan, S.; Chen, B. Experimental Study of Phosphate Salts Influencing Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2014, 65, 480–486. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Liu, C.; Zhang, B.; Chen, H.; Guo, H.; Zhong, G.; Qu, W.; Jiang, S.; Huang, H. Evaluation of Inherent Toxicology and Biocompatibility of Magnesium Phosphate Bone Cement. Colloids Surf. B Biointerfaces 2010, 76, 496–504. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, J.; Chen, H.; Hou, P.; Kawashima, S.; Qin, J.; Zhou, X.; Qian, J.; Cheng, X. Sulfuric Acid-Resistance Performances of Magnesium Phosphate Cements: Macro-Properties, Mineralogy and Microstructure Evolutions. Cem. Concr. Res. 2022, 157, 106830. [Google Scholar] [CrossRef]
- Cao, X.; Lu, H.; Liu, J.; Lu, W.; Guo, L.; Ma, M.; Zhang, B.; Guo, Y. 3D Plotting in the Preparation of Newberyite, Struvite, and Brushite Porous Scaffolds: Using Magnesium Oxide as a Starting Material. J. Mater. Sci. Mater. Med. 2019, 30, 88. [Google Scholar] [CrossRef]
- Liu, N.; Chen, B. Experimental Research on Magnesium Phosphate Cements Containing Alumina. Constr. Build. Mater. 2016, 121, 354–360. [Google Scholar] [CrossRef]
- Iyengar, S.R.; Al-Tabbaa, A. Developmental Study of a Low-PH Magnesium Phosphate Cement for Environmental Applications. Environ. Technol. 2007, 28, 1387–1401. [Google Scholar] [CrossRef]
- Hou, D.; Yan, H.; Zhang, J.; Wang, P.; Li, Z. Experimental and Computational Investigation of Magnesium Phosphate Cement Mortar. Constr. Build. Mater. 2016, 112, 331–342. [Google Scholar] [CrossRef]
- Yang, J.; Qian, C. Effect of Borax on Hydration and Hardening Properties of Magnesium and Pottassium Phosphate Cement Pastes. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2010, 25, 613–618. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, H. Multi-Scale Investigation of Creep Properties of Potassium Magnesium Phosphate Cement. J. Build. Eng. 2022, 48, 103949. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Calorimetric Study of Magnesium Potassium Phosphate Cement. Mater. Struct. 2012, 45, 447–456. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing Mechanism of Potassium Phosphate Based Magnesium Phosphate Cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Chau, C.K.; Qiao, F.; Li, Z. Microstructure of Magnesium Potassium Phosphate Cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- Covill, A.; Hyatt, N.C.; Hill, J.; Collier, N.C. Development of Magnesium Phosphate Cements for Encapsulation of Radioactive Waste. Adv. Appl. Ceram. 2011, 110, 151–156. [Google Scholar] [CrossRef]
- Qian, C.; Yang, J.-M. Effect of Disodium Hydrogen Phosphate on Hydration and Hardening of Magnesium Potassium Phosphate Cement. J. Mater. Civ. Eng. 2011, 23, 1405–1411. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B. Factors That Affect the Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2013, 47, 977–983. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Liu, J.; Pei, H.; Li, Z. Effects of Water Content, Magnesia-to-Phosphate Molar Ratio and Age on Pore Structure, Strength and Permeability of Magnesium Potassium Phosphate Cement Paste. Mater. Des. 2014, 64, 497–502. [Google Scholar] [CrossRef]
- Viani, A.; Peréz-Estébanez, M.; Pollastri, S.; Gualtieri, A.F. In Situ Synchrotron Powder Diffraction Study of the Setting Reaction Kinetics of Magnesium-Potassium Phosphate Cements. Cem. Concr. Res. 2016, 79, 344–352. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the Influence of Mg/P Ratio on the Properties and Durability of Magnesium Potassium Phosphate Cement Pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Platret, G.; Stefan, L. Mechanisms of K-Struvite Formation in Magnesium Phosphate Cements. Cem. Concr. Res. 2017, 91, 117–122. [Google Scholar] [CrossRef]
- Tan, Y.; Dong, J.; Yu, H.; Li, Y.; Wen, J.; Wu, C. Study on the Injectability of a Novel Glucose Modified Magnesium Potassium Phosphate Chemically Bonded Ceramic. Mater. Sci. Eng. C 2017, 79, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ma, H.; Li, Z. Influence of Magnesia-to-Phosphate Molar Ratio on Microstructures, Mechanical Properties and Thermal Conductivity of Magnesium Potassium Phosphate Cement Paste with Large Water-to-Solid Ratio. Cem. Concr. Res. 2015, 68, 1–9. [Google Scholar] [CrossRef]
- Viani, A.; Zbiri, M.; Bordallo, H.N.; Gualtieri, A.F.; Mácová, P. Investigation of the Setting Reaction in Magnesium Phosphate Ceramics with Quasielastic Neutron Scattering. J. Phys. Chem. C 2017, 121, 11355–11367. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Leemann, A.; Winnefeld, F. Reaction Mechanism of Magnesium Potassium Phosphate Cement with High Magnesium-to-Phosphate Ratio. Cem. Concr. Res. 2018, 108, 140–151. [Google Scholar] [CrossRef]
- Lahalle, H.; Cau Dit Coumes, C.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c Ratio on the Hydration Process of a Magnesium Phosphate Cement and on Its Retardation by Boric Acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Property Evaluation of Magnesium Phosphate Cement Mortar as Patch Repair Material. Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Yue, L.; Bing, C. New Type of Super-Lightweight Magnesium Phosphate Cement Foamed Concrete. J. Mater. Civ. Eng. 2015, 27, 04014112. [Google Scholar] [CrossRef]
- Jie, W.; Zhenyu, L.; Xin, H.; Tao, Y.; Mengliang, L.; Qiubai, D.; Khalid, K.; Zhongyuan, L.; Shuzhen, L. Porous Materials Prepared by Magnesium Phosphate Cement for the Effective Immobilization of Lead Ions. Int. J. Environ. Res. 2021, 15, 681–694. [Google Scholar] [CrossRef]
- Zárybnická, L.; Machotová, J.; Mácová, P.; Machová, D.; Viani, A. Design of Polymeric Binders to Improve the Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2021, 290, 123202. [Google Scholar] [CrossRef]
- Zárybnická, L.; Machotová, J.; Mácová, P.; Viani, A. Organic-Inorganic Composites Based on Magnesium Phosphate Cement and Acrylic Latexes: Role of Functional Groups. Ceram. Int. 2023, 49, 4523–4530. [Google Scholar] [CrossRef]
- Ma, C.; Chen, B. Properties of Magnesium Phosphate Cement Containing Redispersible Polymer Powder. Constr. Build. Mater. 2016, 113, 255–263. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, L.; Gong, C.; Li, W.; Zhao, Y.; Guo, W. A Bioactive Magnesium Phosphate Cement Incorporating Chondroitin Sulfate for Bone Regeneration. Biomed. Mater. 2021, 16, 035034. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xia, K.; Gong, C.; Chen, J.; Li, W.; Zhao, Y.; Guo, W.; Dai, H. An Injectable Bioactive Magnesium Phosphate Cement Incorporating Carboxymethyl Chitosan for Bone Regeneration. Int. J. Biol. Macromol. 2020, 160, 101–111. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Kaufmann, J.; Lothenbach, B. Influence of Magnesium-to-Phosphate Ratio and Water-to-Cement Ratio on Hydration and Properties of Magnesium Potassium Phosphate Cements. Cem. Concr. Res. 2019, 123, 105781. [Google Scholar] [CrossRef]
- Viani, A.; Mácová, P.; Ševčík, R.; Zárybnická, L. Mechanism of Magnesium Phosphate Cement Retardation by Citric Acid. Ceram. Int. 2023, 49, 11112–11122. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; He, T. Effects of Sulphoaluminate Cement on the Strength and Water Stability of Magnesium Potassium Phosphate Cement. Constr. Build. Mater. 2017, 132, 335–342. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Sun, S. Effect of M/P and Borax on the Hydration Properties of Magnesium Potassium Phosphate Cement Blended with Large Volume of Fly Ash. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2018, 33, 1159–1167. [Google Scholar] [CrossRef]
- Viani, A.; Mácová, P.; Peréz-Estébanez, M. Nucleation of Amorphous Precursor in Magnesium Phosphate Cements: Clues to the Reaction Pathway. Mater. Lett. 2021, 304, 130677. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Ma, B.; Rentsch, D.; Lothenbach, B. Influence of Aluminum Sulfate on Properties and Hydration of Magnesium Potassium Phosphate Cements. Cem. Concr. Res. 2022, 156, 106788. [Google Scholar] [CrossRef]
- Lai, Z.; Lai, X.; Shi, J.; Lu, Z. Effect of Zn2+ on the Early Hydration Behavior of Potassium Phosphate Based Magnesium Phosphate Cement. Constr. Build. Mater. 2016, 129, 70–78. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Z.; Chen, D.; Li, G.; Chen, J.; Wang, K.; Xu, L.; Huang, J. An Injectable Porous Bioactive Magnesium Phosphate Bone Cement Foamed with Calcium Carbonate and Citric Acid for Periodontal Bone Regeneration. J. Mech. Behav. Biomed. Mater. 2023, 142, 105805. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhang, J.; Li, J.; Ma, A.; Liu, L. Effect of Liquid-to-Solid Ratios on the Properties of Magnesium Phosphate Chemically Bonded Ceramics. Mater. Sci. Eng. C 2013, 33, 2508–2512. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Yan, Z.; Chen, Z. High-Temperature Resistance of Modified Potassium Magnesium Phosphate Cement. Materials 2022, 15, 8967. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, F.; Guo, J.; Yao, K. Properties and Reaction Mechanisms of Magnesium Phosphate Cement Mixed with Ferroaluminate Cement. Materials 2019, 12, 2561. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Lothenbach, B.; Winnefeld, F. Influence of Wollastonite on Hydration and Properties of Magnesium Potassium Phosphate Cements. Cem. Concr. Res. 2020, 131, 106012. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Lothenbach, B. Effect of Temperature Curing on Properties and Hydration of Wollastonite Blended Magnesium Potassium Phosphate Cements. Cem. Concr. Res. 2021, 142, 106370. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Li, Z. Properties and Hydrates of Seawater-Mixed Magnesium Potassium Phosphate Cements with High Magnesium-to-Phosphate Ratio. Cem. Concr. Compos. 2022, 134, 104807. [Google Scholar] [CrossRef]
- Chong, L.; Shi, C.; Yang, J.; Jia, H. Effect of Limestone Powder on the Water Stability of Magnesium Phosphate Cement-Based Materials. Constr. Build. Mater. 2017, 148, 590–598. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Chen, Z.; Kang, T.; Ding, X.; Li, Y.; Liao, Y.; Chen, C.; Yuan, H.; Peng, H. A Study on Bone Cement Containing Magnesium Potassium Phosphate for Bone Repair. Cogent Biol. 2018, 4, 1487255. [Google Scholar] [CrossRef]
- Han, Z.; Wang, B.; Ren, B.; Liu, Y.; Zhang, N.; Wang, Z.; Liu, J.; Mao, K. Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement. Life 2022, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yuan, Z.; Zhang, J.; Liu, L.; Li, J.; Liu, Z. Effect of Raw Material Ratios on the Compressive Strength of Magnesium Potassium Phosphate Chemically Bonded Ceramics. Mater. Sci. Eng. C 2013, 33, 5058–5063. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Lai, Z.; Luo, X.; Liu, Z.; Liu, M.; Deng, Q.; Chen, J.; Lu, Z.; Lv, S. Effects of Sodium Dihydrogen Phosphate on Properties of Magnesium Phosphate Cement. J. Build. Eng. 2022, 60, 105216. [Google Scholar] [CrossRef]
- Feng, H.; Li, Z.; Wang, W.; Liu, G.; Zhang, Z.; Gao, D. Deflection Hardening Behaviour of Ductile Fibre Reinforced Magnesium Phosphate Cement-Based Composite. Cem. Concr. Compos. 2021, 121, 104079. [Google Scholar] [CrossRef]
- Jeon, I.K.; Qudoos, A.; Kim, H.G. Influence of Carbonation Curing on Hydration and Microstructure of Magnesium Potassium Phosphate Cement Concrete. J. Build. Eng. 2021, 38, 102203. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, G.; Li, Y. Study on Graphene Oxide Reinforced Magnesium Phosphate Cement Composites. Constr. Build. Mater. 2022, 359, 129523. [Google Scholar] [CrossRef]
- Gong, C.; Fang, S.; Xia, K.; Chen, J.; Guo, L.; Guo, W. Enhancing the Mechanical Properties and Cytocompatibility of Magnesium Potassium Phosphate Cement by Incorporating Oxygen-Carboxymethyl Chitosan. Regen. Biomater. 2021, 8, rbaa048. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, X.; Pei, J.; Zhao, D.; Yan, Y. Fabrication of Magnesium Phosphate Bone Cement with Enhanced Osteogenic Properties by Employing Zeolitic Imidazolate Framework-8. J. Mater. Res. 2022, 37, 2761–2774. [Google Scholar] [CrossRef]
- Wang, M.; Liang, L.; Liu, Q.; Liang, X.; Guo, H.; Li, Z.; Liang, R.; Sun, G. Influence of Dipotassium Hydrogen Phosphate on Properties of Magnesium Potassium Phosphate Cement. Constr. Build. Mater. 2022, 320, 126283. [Google Scholar] [CrossRef]
- Li, Y.; Liao, W.; Taghvaee, T.; Wu, C.; Ma, H.; Leventis, N. Bioinspired Strong Nanocellular Composite Prepared with Magnesium Phosphate Cement and Polyurea Aerogel. Mater. Lett. 2019, 237, 274–277. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Rousselet, A.; Xu, B.; Mercier, C.A.; Gauffinet, S. Investigation of Aluminum Nitrate as a Set Retarder of Magnesium Potassium Phosphate Cement: Mechanisms Involved in Diluted Suspension. Cem. Concr. Res. 2021, 150, 106608. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Mu, B.; Wang, A. Incorporation of Clay Minerals into Magnesium Phosphate Bone Cement for Enhancing Mechanical Strength and Bioactivity. Biomed. Mater. 2023, 18, 025002. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gao, T.; Li, W.; Yang, J.; Liu, Y.; Zhao, Y.; He, P.; Li, X.; Guo, W.; Fan, Z.; et al. Carboxymethyl Chitosan-Alginate Enhances Bone Repair Effects of Magnesium Phosphate Bone Cement by Activating the FAK-Wnt Pathway. Bioact. Mater. 2023, 20, 598–609. [Google Scholar] [CrossRef]
- Zhou, H.; Agarwal, A.K.; Goel, V.K.; Bhaduri, S.B. Microwave Assisted Preparation of Magnesium Phosphate Cement (MPC) for Orthopedic Applications: A Novel Solution to the Exothermicity Problem. Mater. Sci. Eng. C 2013, 33, 4288–4294. [Google Scholar] [CrossRef]
- Klammert, U.; Vorndran, E.; Reuther, T.; Müller, F.A.; Zorn, K.; Gbureck, U. Low Temperature Fabrication of Magnesium Phosphate Cement Scaffolds by 3D Powder Printing. J. Mater. Sci. Mater. Med. 2010, 21, 2947–2953. [Google Scholar] [CrossRef]
- Ewald, A.; Helmschrott, K.; Knebl, G.; Mehrban, N.; Grover, L.M.; Gbureck, U. Effect of Cold-Setting Calcium- and Magnesium Phosphate Matrices on Protein Expression in Osteoblastic Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 326–332. [Google Scholar] [CrossRef]
- Großardt, C.; Ewald, A.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Passive and Active In Vitro Resorption of Calcium and Magnesium Phosphate Cements by Osteoclastic Cells. Tissue Eng. Part A 2010, 16, 3687–3695. [Google Scholar] [CrossRef]
- Ewald, A.; Lochner, B.; Gbureck, U.; Groll, J.; Krüger, R. Structural Optimization of Macroporous Magnesium Phosphate Scaffolds and Their Cytocompatibility. Key Eng. Mater. 2012, 493–494, 813–819. [Google Scholar] [CrossRef]
- Moseke, C.; Saratsis, V.; Gbureck, U. Injectability and Mechanical Properties of Magnesium Phosphate Cements. J. Mater. Sci. Mater. Med. 2011, 22, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Wunder, K.; Moseke, C.; Biermann, I.; Müller, F.A.; Zorn, K.; Gbureck, U. Hydraulic Setting Mg3(PO4)2 Powders for 3D Printing Technology. Adv. Appl. Ceram. 2011, 110, 476–481. [Google Scholar] [CrossRef]
- Lee, J.; Farag, M.M.; Park, E.K.; Lim, J.; Yun, H. A Simultaneous Process of 3D Magnesium Phosphate Scaffold Fabrication and Bioactive Substance Loading for Hard Tissue Regeneration. Mater. Sci. Eng. C 2014, 36, 252–260. [Google Scholar] [CrossRef]
- Christel, T.; Christ, S.; Barralet, J.E.; Groll, J.; Gbureck, U. Chelate Bonding Mechanism in a Novel Magnesium Phosphate Bone Cement. J. Am. Ceram. Soc. 2015, 98, 694–697. [Google Scholar] [CrossRef]
- Gelli, R.; Mati, L.; Ridi, F.; Baglioni, P. Tuning the Properties of Magnesium Phosphate-Based Bone Cements: Effect of Powder to Liquid Ratio and Aqueous Solution Concentration. Mater. Sci. Eng. C 2019, 95, 248–255. [Google Scholar] [CrossRef]
- Ostrowski, N.; Sharma, V.; Roy, A.; Kumta, P.N. Systematic Assessment of Synthesized Tri-Magnesium Phosphate Powders (Amorphous, Semi-Crystalline and Crystalline) and Cements for Ceramic Bone Cement Applications. J. Mater. Sci. Technol. 2015, 31, 437–444. [Google Scholar] [CrossRef]
- Kim, J.-A.; Lim, J.; Naren, R.; Yun, H.; Park, E.K. Effect of the Biodegradation Rate Controlled by Pore Structures in Magnesium Phosphate Ceramic Scaffolds on Bone Tissue Regeneration in Vivo. Acta Biomater. 2016, 44, 155–167. [Google Scholar] [CrossRef]
- Brückner, T.; Hurle, K.; Stengele, A.; Groll, J.; Gbureck, U. Mechanical Activation and Cement Formation of Trimagnesium Phosphate. J. Am. Ceram. Soc. 2018, 101, 1830–1834. [Google Scholar] [CrossRef]
- Farag, M.M.; Yun, H. Effect of Gelatin Addition on Fabrication of Magnesium Phosphate-Based Scaffolds Prepared by Additive Manufacturing System. Mater. Lett. 2014, 132, 111–115. [Google Scholar] [CrossRef]
- Tonelli, M.; Gelli, R.; Ridi, F.; Baglioni, P. Magnesium Phosphate-Based Cements Containing Halloysite Nanotubes for Cracks Repair. Constr. Build. Mater. 2021, 301, 124056. [Google Scholar] [CrossRef]
- Kanter, B.; Vikman, A.; Brückner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone Regeneration Capacity of Magnesium Phosphate Cements in a Large Animal Model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef]
- Kim, J.A.; Yun, H.; Choi, Y.-A.; Kim, J.-E.; Choi, S.-Y.; Kwon, T.-G.; Kim, Y.K.; Kwon, T.-Y.; Bae, M.A.; Kim, N.J.; et al. Magnesium Phosphate Ceramics Incorporating a Novel Indene Compound Promote Osteoblast Differentiation in Vitro and Bone Regeneration in Vivo. Biomaterials 2018, 157, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gelli, R.; Di Pompo, G.; Graziani, G.; Avnet, S.; Baldini, N.; Baglioni, P.; Ridi, F. Unravelling the Effect of Citrate on the Features and Biocompatibility of Magnesium Phosphate-Based Bone Cements. ACS Biomater. Sci. Eng. 2020, 6, 5538–5548. [Google Scholar] [CrossRef]
- Kaiser, F.; Schröter, L.; Stein, S.; Krüger, B.; Weichhold, J.; Stahlhut, P.; Ignatius, A.; Gbureck, U. Accelerated Bone Regeneration through Rational Design of Magnesium Phosphate Cements. Acta Biomater. 2022, 145, 358–371. [Google Scholar] [CrossRef]
- Gelli, R.; Sforzi, L.; Montanari, F.; Ridi, F.; Baglioni, P. Alendronate-Loaded Gelatin Microparticles as Templating Agents for Macroporous Magnesium Phosphate-Based Bone Cements. J. Mater. Sci. 2022, 57, 12994–13010. [Google Scholar] [CrossRef]
- Gelli, R.; Tonelli, M.; Martini, F.; Calucci, L.; Borsacchi, S.; Ridi, F. Effect of Borax on the Hydration and Setting of Magnesium Phosphate Cements. Constr. Build. Mater. 2022, 348, 128686. [Google Scholar] [CrossRef]
- Liu, J.; Hou, W.; Wei, W.; Peng, J.; Wu, X.; Lian, C.; Zhao, Y.; Tu, R.; Goto, T.; Dai, H. Design and Fabrication of High-Performance Injectable Self-Setting Trimagnesium Phosphate. Bioact. Mater. 2023, 28, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Brückner, T.; Meininger, M.; Groll, J.; Kübler, A.C.; Gbureck, U. Magnesium Phosphate Cement as Mineral Bone Adhesive. Materials 2019, 12, 3819. [Google Scholar] [CrossRef] [Green Version]
- Heilig, P.; Sandner, P.; Jordan, M.C.; Jakubietz, R.G.; Meffert, R.H.; Gbureck, U.; Hoelscher-Doht, S. Experimental Drillable Magnesium Phosphate Cement Is a Promising Alternative to Conventional Bone Cements. Materials 2021, 14, 1925. [Google Scholar] [CrossRef]
- Heilig, P.; Jordan, M.C.; Paul, M.M.; Kupczyk, E.; Meffert, R.H.; Gbureck, U.; Hoelscher-Doht, S. Augmentation of Suture Anchors with Magnesium Phosphate Cement—Simple Technique with Striking Effect. J. Mech. Behav. Biomed. Mater. 2022, 128, 105096. [Google Scholar] [CrossRef]
- Babaie, E.; Lin, B.; Goel, V.K.; Bhaduri, S.B. Evaluation of Amorphous Magnesium Phosphate (AMP) Based Non-Exothermic Orthopedic Cements. Biomed. Mater. 2016, 11, 055010. [Google Scholar] [CrossRef]
- Strydom, C.A.; van der Merwe, E.M.; Aphane, M.E. The Effect of Calcining Conditions on the Rehydration of Dead Burnt Magnesium Oxide Using Magnesium Acetate as a Hydrating Agent. J. Therm. Anal. Calorim. 2005, 80, 659–662. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Smirnov, V.V.; Krokhicheva, P.A.; Barinov, S.M.; Komlev, V.S. The Creation and Application Outlook of Calcium Phosphate and Magnesium Phosphate Bone Cements with Antimicrobial Properties (Review). Inorg. Mater. Appl. Res. 2021, 12, 195–203. [Google Scholar] [CrossRef]
- Wagh, A.S.; Jeong, S.Y. Chemically Bonded Phosphate Ceramics: I, A Dissolution Model of Formation. J. Am. Ceram. Soc. 2003, 86, 1838–1844. [Google Scholar] [CrossRef]
- Han, W.; Chen, H.; Li, X.; Zhang, T. Thermodynamic Modeling of Magnesium Ammonium Phosphate Cement and Stability of Its Hydration Products. Cem. Concr. Res. 2020, 138, 106223. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, H.-W.; Ginebra, M.-P. Polymeric Additives to Enhance the Functional Properties of Calcium Phosphate Cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef]
- Khairoun, I.; Driessens, F.C.M.; Boltong, M.G.; Planell, J.A.; Wenz, R. Addition of Cohesion Promotors to Calcium Phosphate Cements. Biomaterials 1999, 20, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Doebelin, N.; Baroud, G. Theoretical and Experimental Approach to Test the Cohesion of Calcium Phosphate Pastes. Eur. Cells Mater. 2006, 12, 26–35. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Mariño, F.T.; Torres, J.; Jerez, L.B.; Gbureck, U.; Cabarcos, E.L. The Effect of Hyaluronic Acid on Brushite Cement Cohesion. Acta Biomater. 2009, 5, 3150–3156. [Google Scholar] [CrossRef]
- An, J.; Wolke, J.G.C.; Jansen, J.A.; Leeuwenburgh, S.C.G. Influence of Polymeric Additives on the Cohesion and Mechanical Properties of Calcium Phosphate Cements. J. Mater. Sci. Mater. Med. 2016, 27, 58. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M.; Gbureck, U.; Barralet, J.E. Technological Issues for the Development of More Efficient Calcium Phosphate Bone Cements: A Critical Assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.O. Vertebral Body Reconstruction: Review and Update on Vertebroplasty and Kyphoplasty. Appl. Radiol. 2008, 37, 10–24. [Google Scholar] [CrossRef]
- Khan, A.; Mirza, E.; Mohamed, B.; ElSharawy, M.; Al-Asmari, M.; Al-Khureif, A.; Dar, M.; Vallittu, P. Static and Dynamic Mechanical Properties of Graphene Oxide-Based Bone Cementing Agents. J. Compos. Mater. 2019, 53, 002199831982634. [Google Scholar] [CrossRef]
- Canal, C.; Ginebra, M.P. Fibre-Reinforced Calcium Phosphate Cements: A Review. J. Mech. Behav. Biomed. Mater. 2011, 4, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of Ceramic Materials as Permanently Implantable Skeletal Prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-Setting Bioactive Calcium–Magnesium Phosphate Cement with High Strength and Degradability for Bone Regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef]
- Wu, F.; Su, J.; Wei, J.; Guo, H.; Liu, C. Injectable Bioactive Calcium–Magnesium Phosphate Cement for Bone Regeneration. Biomed. Mater. 2008, 3, 044105. [Google Scholar] [CrossRef]
- Egorov, A.A.; Fedotov, A.Y.; Pereloma, I.S.; Teterina, A.Y.; Sergeeva, N.S.; Sviridova, I.K.; Kirsanova, V.A.; Akhmedova, S.A.; Nesterova, A.V.; Reshetov, I.V.; et al. Calcium Phosphate Composite Cements Based on Simple Mixture of Brushite and Apatite Phases. IOP Conf. Ser. Mater. Sci. Eng. 2018, 347, 012039. [Google Scholar] [CrossRef]
- Gelli, R.; Scudero, M.; Gigli, L.; Severi, M.; Bonini, M.; Ridi, F.; Baglioni, P. Effect of PH and Mg2+ on Amorphous Magnesium-Calcium Phosphate (AMCP) Stability. J. Colloid Interface Sci. 2018, 531, 681–692. [Google Scholar] [CrossRef]
- Wang, A.; Song, N.; Fan, X.; Li, J.; Bai, L.; Song, Y.; He, R. Characterization of Magnesium Phosphate Cement Fabricated Using Pre-Reacted Magnesium Oxide. J. Alloys Compd. 2017, 696, 560–565. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Hou, D.; Wang, Z. Nanoscale Insight on the Initial Hydration Mechanism of Magnesium Phosphate Cement. Constr. Build. Mater. 2021, 276, 122213. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Health and Consumers. In Opinion on Boron Compounds; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Li, Y.; Lin, H.; Hejazi, S.M.A.S.; Zhao, C.; Xie, M. The Effect of Low Temperature Phase Change Material of Hydrated Salt on the Performance of Magnesium Phosphate Cement. Constr. Build. Mater. 2017, 149, 272–278. [Google Scholar] [CrossRef]
- Yu, S.; Liu, L.; Xu, C.; Dai, H. Magnesium Phosphate Based Cement with Improved Setting, Strength and Cytocompatibility Properties by Adding Ca(H2PO4)2·H2O and Citric Acid. J. Mech. Behav. Biomed. Mater. 2019, 91, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Tran, R.T.; Yang, J.; Ameer, G.A. Citrate-Based Biomaterials and Their Applications in Regenerative Engineering. Annu. Rev. Mater. Res. 2015, 45, 277–310. [Google Scholar] [CrossRef]
- Jehle, S.; Hulter, H.N.; Krapf, R. Effect of Potassium Citrate on Bone Density, Microarchitecture, and Fracture Risk in Healthy Older Adults without Osteoporosis: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Granchi, D.; Caudarella, R.; Ripamonti, C.; Spinnato, P.; Bazzocchi, A.; Massa, A.; Baldini, N. Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2018, 10, 1293. [Google Scholar] [CrossRef] [Green Version]
- Zárybnická, L.; Mácová, P.; Viani, A. Properties Enhancement of Magnesium Phosphate Cement by Cross-Linked Polyvinyl Alcohol. Ceram. Int. 2022, 48, 1947–1955. [Google Scholar] [CrossRef]
- Granchi, D.; Baldini, N.; Ulivieri, F.M.; Caudarella, R. Role of Citrate in Pathophysiology and Medical Management of Bone Diseases. Nutrients 2019, 11, 2576. [Google Scholar] [CrossRef] [Green Version]
- Gelli, R.; Bernardini, G.; Ridi, F. Strontium-Loaded Magnesium Phosphate Bone Cements and Effect of Polymeric Additives. Ceram. Int. 2023, 49, 31466–31476. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xu, J.-K.; Hopkins, C.; Chow, D.H.-K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium Deficiency: Effect on Bone and Mineral Metabolism in the Mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium Ion Stimulation of Bone Marrow Stromal Cells Enhances Osteogenic Activity, Simulating the Effect of Magnesium Alloy Degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luthringer, B.J.C.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of Extracellular Magnesium on the Differentiation and Function of Human Osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, N.; Lee, B.; Hong, D.; Enick, P.N.; Roy, A.; Kumta, P.N. Synthesis, Osteoblast, and Osteoclast Viability of Amorphous and Crystalline Tri-Magnesium Phosphate. ACS Biomater. Sci. Eng. 2015, 1, 52–63. [Google Scholar] [CrossRef]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Blanco, L.; López-Cabarcos, E. Magnesium Substitution in Brushite Cements for Enhanced Bone Tissue Regeneration. Mater. Sci. Eng. C 2014, 43, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, H.; Wei, J.; Jiang, X.; Hua, H.; Chen, F.; Wei, S.; Shin, J.-W.; Liu, C. Development of Magnesium Calcium Phosphate Biocement for Bone Regeneration. J. R. Soc. Interface 2010, 7, 1171–1180. [Google Scholar] [CrossRef]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In Vivo Degradation of Low Temperature Calcium and Magnesium Phosphate Ceramics in a Heterotopic Model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef]
- Zeng, D.; Xia, L.; Zhang, W.; Huang, H.; Wei, B.; Huang, Q.; Wei, J.; Liu, C.; Jiang, X. Maxillary Sinus Floor Elevation Using a Tissue-Engineered Bone with Calcium-Magnesium Phosphate Cement and Bone Marrow Stromal Cells in Rabbits. Tissue Eng. Part A 2012, 18, 870–881. [Google Scholar] [CrossRef]
- Schendel, S.A.; Peauroi, J. Magnesium-Based Bone Cement and Bone Void Filler: Preliminary Experimental Studies. J. Craniofacial Surg. 2009, 20, 461. [Google Scholar] [CrossRef]
- Hirvinen, L.J.M.; Litsky, A.S.; Samii, V.F.; Weisbrode, S.E.; Bertone, A.L. Influence of Bone Cements on Bone-Screw Interfaces in the Third Metacarpal and Third Metatarsal Bones of Horses. Am. J. Vet. Res. 2009, 70, 964–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehlke, B.M.; Wilson, T.G.; Jones, A.A.; Yamashita, M.; Cochran, D.L. The Use of a Magnesium-Based Bone Cement to Secure Immediate Dental Implants. Oral Craniofacial Tissue Eng. 2013, 28, e357–e367. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Chemical Formula | Mineral Name | Solubility (Ksp) | Mg/P Atomic Ratio | Abbreviation |
|---|---|---|---|---|
| Mg3(PO4)2 | Farringtonite | 3.9 × 10−23 [50] | 1.5 | TMP |
| Mg3(PO4)2·8H2O | Bobierrite | 6.3 × 10−26 [51] | 1.5 | BOB |
| Mg3(PO4)2·22H2O | Cattiite 1 | 8 × 10−24 [51] | 1.5 | CAT |
| MgNH4PO4·H2O | Dittmarite | Unknown | 1 | DIT |
| MgHPO4·3H2O | Newberyite | 1.5 × 10−6 [51] | 1 | NEW |
| MgHPO4·7H2O | Phosphorrösslerite | 9.77 × 10−18 [50] | 1 | PHO |
| MgNH4PO4·6H2O | Struvite | 7.1 × 10−14 [52] | 1 | STR |
| MgKPO4·6H2O | K- Struvite | 2.4 × 10−11 [52] | 1 | KST |
| (NH4)2Mg3(HPO4)4·8H2O | Hannayite 2 | Unknown | 0.75 | HAN |
| (NH4)2Mg(HPO4)2·4H2O | Schertelite 2 | Unknown | 0.5 | SCH |
| Powder Component | Phosphate Salt Aqueous Solution | Additional Components | P/L 1 | Final Crystalline Phases | References |
|---|---|---|---|---|---|
| MgO | NH4H2PO4 | - | - | STR + SCH + DIT | [54]—N |
| MgO | NH4H2PO4 | Silica | 8.3–20 g/mL | MgO + STR | [55]—N |
| MgO | NH4H2PO4 | Sand | 4 g/mL | MgO + STR | [56]—C |
| MgO | NH4H2PO4 + NaH2PO4 | Borax | 7.7 g/mL | MgO + STR + SCH | [57]—B |
| MgO | NH4H2PO4 + NaH2PO4 | Borax, Bi2O3 | 7.7 g/mL | MgO + STR + SCH | [58]—B |
| MgO | NH4H2PO4 | Borax + Zn + quartz | 5.6–7.1 g/mL | - | [59]—C |
| MgO | NH4H2PO4 + NaH2PO4 | Chitosan | 9 g/mL | MgO + STR + SCH + Na2Mg(HPO4)2 | [60]—B |
| MgO | NH4H2PO4 | Na2B4O7·5H2O | 8.3 g/mL | MgO + STR + DIT | [61]—N |
| MgO | NH4H2PO4 | Borax, acetic acid | 8.3 g/mL | MgO + STR + Mg(acetate) + NH4H2PO4 | [62]—C |
| MgO | NH4H2PO4 + NaH2PO4 | Borax | 7.7 g/mL | MgO + STR + SCH | [63]—B |
| MgO | NH4H2PO4 | Borax, acetic acid, sand | 8.3 g/mL | MgO + STR | [64]—C |
| MgO | NH4H2PO4 | Borax, sand, metakaolin | 4.3 g/mL | MgO + STR | [65]—C |
| MgO | NH4H2PO4 | Borax, Na5P3O10, metakaolin, sand, sodium polyacrylate | 5 g/mL | STR | [66]—C |
| MgO | NH4H2PO4 | Borax, Na5P3O10, acetic acid | 6.66 g/mL | MgO + STR | [67]—C |
| MgO | NH4H2PO4 | H3BO3 | - | MgO + STR + DIT | [68]—N |
| MgO + AMP | PVA solution | Mg granules | 0.5 g/mL | BOB | [69]—B |
| MgO | NH4H2PO4 | Na5P3O10, H3BO3, sand | 5.3–6.3 g/mL | - | [70]—C |
| MgO | NH4H2PO4 | Na5P3O10, borax, H3BO3, sand | - | - | [71]—C |
| MgO | NH4H2PO4 | Metakaolin, Al2O3, borax, sand | 11.1 g/mL | MgO + STR + Al phosphate hydrates | [72]—C |
| MgO | NH4H2PO4 | Borax, citric acid, quartz, foaming agent | 5.5–7.1 g/mL | MgO + STR + SiO2 | [73]—C |
| MgO | NH4H2PO4 | H3BO3 | 1.7 g/mL | MgO + STR | [74]—N |
| MgO | NH4H2PO4 | Fly ash, sand, borax, Na5P3O10 | 5 g/mL | MgO + STR | [75]—C |
| MgO | NH4H2PO4 | Borax, H2O2, Al2O3, zeolite | 2.1–2.6 g/mL | MgO + STR + NH4H2PO4 | [76]—N |
| MgO | H3PO4 | MgCl2 | 0.6–1 g/mL | Mg(OH)2·MgCl2·8H2O | [77]—B |
| MgO | KH2PO4 +NH4H2PO4 | Fly ash, borax, Na5P3O10, | 10 g/mL | MgO + STR + KST | [78]—C |
| MgO | NH4H2PO4 | retarder | - | - | [79]—B |
| MgO | KH2PO4, NH4H2PO4 | Borax | 7.1 g/mL | MgO + STR/KST + DIT | [80]—C |
| MgO | KH2PO4, NH4H2PO4 | Pluronic F127 | - | MgO, Mg(OH)2, STR, NEW | [81]—B |
| MgO | KH2PO4 + NH4H2PO4 | Al2O3, borax | 6.25–7.1 g/mL | MgO + STR + KST + alumina phosphate hydrate | [82]—N |
| MgO | KH2PO4 + NH4H2PO4 | H3BO3, Na5P3O10 | - | MgO + KST + KH2PO4 + lünebergite | [83]—C |
| MgO | KH2PO4, NaH2PO4 | Borax, sand | 5 g/mL | MgO + KST + NaST | [84]—C |
| MgO | KH2PO4 | Borax | 8.3–12.5 g/mL | MgO + KST + borax | [85]—C |
| MgO | KH2PO4 | Borax | 5–7.1 g/mL | MgO + KST | [86]—C |
| MgO | KH2PO4 | Borax | 0.1–0.4 g/mL | MgO + KST | [87]—C |
| MgO | KH2PO4 | - | 6.66 g/mL | MgO + KST | [88]—C |
| MgO | KH2PO4 | Borax | - | MgO + KST | [89]—C |
| MgO | KH2PO4 | Fly ash, H3BO3 | 3.6–3.8 g/mL | MgO + KST + SiO2 | [90]—C |
| MgO | KH2PO4, Na2HPO4·12H2O | Borax | - | MgO + KST + Na2Mg(HPO4)2 + K2Mg(HPO4)2·4H2O | [91]—C |
| MgO | KH2PO4 | Fly ash, borax, sand | 5–7.1 g/mL | MgO + KST | [92]—C |
| MgO | KH2PO4 | - | 2–6.66 g/mL | MgO + KST | [93]—C |
| MgO | KH2PO4 | - | 3.3 g/mL | MgO + KST | [94]—N |
| MgO | KH2PO4 | - | 5 g/mL | MgO + KST | [95,96]—C |
| MgO | KH2PO4 | Glucose | 3.3–5.5 g/mL | MgO + KST | [97]—B |
| MgO | KH2PO4 | - | 2 g/mL | MgO + KST | [98]—C |
| MgO | KH2PO4 | - | 2.3 g/mL | MgO + KST | [99]—N |
| MgO | KH2PO4 | - | 2 g/mL | MgO + KST | [100]—N |
| MgO | KH2PO4 | H3BO3 | 1 g/mL | Mg2KH(PO4)2·15H2O + KST | [101]—N |
| MgO | KH2PO4 | Borax, fly ash, sand | 4.3–6.2 g/mL | MgO + KST + SiO2 | [102]—C |
| MgO | KH2PO4 | Sand, fly ash, | 3.3 g/mL | - | [103]—C |
| MgO | KH2PO4 | Borax, NaHCO3 | 5.9 g/mL | MgO + KST | [104]—C |
| MgO | KH2PO4 | Acylic latexes | 2.3 g/mL | MgO + KST | [105,106]—C |
| MgO | KH2PO4 | Silanes, borax, Na5P3O10 | 9 g/mL | MgO + KST | [107]—C |
| MgO | KH2PO4 | Chondroitin sulfate | 2 g/mL | MgO + KST | [108]—B |
| MgO | KH2PO4 | Carboxymethyl chitosan | 2 g/mL | MgO + KST | [109]—B |
| MgO | KH2PO4 | - | 0.2–4 g/mL | MgO + KST+ PHO + Mg2KH(PO4)2·15H2O | [110]—C |
| MgO | KH2PO4 | Citric acid | 2 g/mL | MgO + KST | [111]—N |
| MgO | KH2PO4 | Borax, sand, sulphoaluminate cement | 6.25 g/mL | MgO + KST + Ca-phases | [112]—C |
| MgO | KH2PO4 | Borax, fly ash | 7.14 g/mL | MgO + KST | [113]—C |
| MgO | KH2PO4 | - | 2.3 g/mL | MgO + KST | [114]—N |
| MgO | KH2PO4 | Al2(SO4)3·16H2O | 0.2–4 g/mL | MgO + KST + others | [115]—C |
| MgO | KH2PO4 | Borax, Zn(NO3)2 | 0.1–7.14 g/mL | MgO + KST | [116]—C |
| MgO | KH2PO4 | CaCO3, citric acid | 0.16 g/mL | MgO + KST + CaCO3 | [117]—B |
| MgO | KH2PO4 | - | 0.25–0.7 g/mL | MgO + KST | [118]—B |
| MgO | KH2PO4 | Borax, fly ash, silica fume, sand | 5.5 g/mL | - | [119]—C |
| MgO | KH2PO4 | Borax, ferroaluminates | 4.5 g/mL | MgO + KST + aluminates | [120]—C |
| MgO | KH2PO4 | CaSiO3, quartz, MgCl2 | 1.2–4 g/mL | MgO + KST + CaSiO3 + Mg2KH(PO4)2·15H2O | [121]—C |
| MgO | KH2PO4 | CaSiO3 | 4 g/mL | MgO + KST + BOB + MgKPO4·H2O + CaSiO3 | [122]—C |
| MgO | KH2PO4 | - | 6.7 g/mL | MgO + KST + BOB | [123]—C |
| MgO | KH2PO4 | Borax, limestone, Na2HPO4·12H2O | 6.25 g/mL | MgO + KST + limestone | [124]—C |
| MgO | KH2PO4 + H3PO4 | Sucrose, Na5P3O10, hydroxyapatite | 3.3 g/mL | MgO + KST | [125]—B |
| MgO | KH2PO4 | Sucrose, borax, hydroxyapatite | 6.25 g/mL | MgO + KST | [126]—B |
| MgO | KH2PO4 | - | 6 g/mL | MgO + KST | [127]—B |
| MgO | KH2PO4 | Borax, NaH2PO4 | 5 g/mL | Na-KST | [128]—C |
| MgO | KH2PO4 | Borax, fly ash, sand, PVA fibers | 5.3–10 g/mL | - | [129]—C |
| MgO | KH2PO4 | Borax, sand, basalt | 2.2 g/mL | MgO + KST + MgCO3 + MgCO3·3H2O | [130]—C |
| MgO | KH2PO4 | Graphene oxide, borax, SDS | 7.14 g/mL | MgO + KST | [131]—C |
| MgO | KH2PO4 | Oxygen-carboxymethyl chitosan | 2 g/mL | MgO + KST | [132]—B |
| MgO | KH2PO4 | ZIF-8 | 2.5 g/mL | MgO + KST | [133]—B |
| MgO | KH2PO4, K2HPO4·3H2O | - | 2.7 g/mL | MgO + KST+ MgKPO4·H2O | [134]—C |
| MgO | KH2PO4 | H3BO3, polyurea aerogel | - | - | [135]—B |
| MgO | KH2PO4 | H3BO3, Al(NO3)3 | 0.01 g/mL | CAT + NEW | [136]—C |
| MgO | KH2PO4 | Laponite, sepiolite, halloysite | 2 g/mL | MgO + KST | [137]—B |
| MgO | KH2PO4 | Carboxymethyl chitosan, sodium alginate | 2 g/mL | MgO + KST | [138]—B |
| Mg(OH)2 | H3PO4 | Microwaves | 2.5 g/mL | NEW | [139]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 3 g/mL | TMP + STR + NEW | [140]—B |
| TMP + STR | (NH4)2HPO4 | - | 2.1 g/mL | TMP + STR | [141,142]—B |
| BOB | (NH4)2HPO4 | TWEEN20, PU foam | - | TMP + STR | [143]—B |
| TMP | (NH4)2HPO4 | (NH4)2C6H6O7 | 1–3.3 g/mL | TMP + STR | [144]—B |
| TMP | (NH4)2HPO4/K2HPO4/ H3PO4 | HPMC | 2 g/mL | STR, KST, NEW | [145]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 2–3 g/mL | TMP + STR | [46]—B |
| TMP | (NH4)2HPO4 | HPMC | 1.5 g/mL | TMP + STR | [146]—B |
| TMP | Phytic acid | 3 g/mL | TMP + NEW | [147]—B | |
| TMP | (NH4)2HPO4 | - | 1–2 g/mL | TMP + STR | [148]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 0.33–2 g/mL | HAN | [149]—B |
| TMP | (NH4)2HPO4 | HPMC + NaCl | 1.33 g/mL | TMP + STR | [150]—B |
| TMP 2 | H2O | - | 1.5 g/mL | TMP + CAT | [151]—B |
| TMP | (NH4)2HPO4 | HPMC + gelatin | - | STR | [152]—B |
| TMP | (NH4)2HPO4 | Halloysite | 1.5 g/mL | TMP + STR | [153]—C |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 2–3 g/mL | TMP + STR | [154]—B |
| TMP | (NH4)2HPO4 | HPMC + indene | 1.33 g/mL | TMP + STR | [155]—B |
| TMP | (NH4)2HPO4 | ammonium citrate | 2 g/mL | TMP + STR | [156]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4/K2HPO4 + KH2PO4 | - | 0.6–1.5 g/mL | TMP + STR + NEW + SCH + KST | [157]—B |
| TMP | (NH4)2HPO4 | Gelatin microparticles | 1.5 g/mL | TMP + STR | [158]—B |
| TMP | (NH4)2HPO4 | Borax | 1.5 g/mL | TMP + STR | [159]—N |
| TMP | KH2PO4 | - | 2–3 g/mL | TMP + KST + NEW | [160]—B |
| MgO+ TMP | Phytic acid | - | 2 g/mL | TMP + NEW | [161]—B |
| MgO+ TMP | Phytic acid | - | 1.71–2 g/mL | MgO + TMP + NEW | [162,163]—B |
| AMP | H2O | PVA | 0.4–0.6 g/mL | CAT | [164]—B |
| Location | Mg Content (g) | % Mg in Total |
|---|---|---|
| Bone | 12.720 | 60–65% |
| Muscle | 6.480 | 27% |
| Other cells | 4.608 | 6–7% |
| Erythrocites | 0.120 | 0.5% |
| Serum | 0.072 | 0.3% |
| Extracellular | - | <1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelli, R.; Ridi, F. An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials. J. Funct. Biomater. 2023, 14, 424. https://doi.org/10.3390/jfb14080424
Gelli R, Ridi F. An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials. Journal of Functional Biomaterials. 2023; 14(8):424. https://doi.org/10.3390/jfb14080424
Chicago/Turabian StyleGelli, Rita, and Francesca Ridi. 2023. "An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials" Journal of Functional Biomaterials 14, no. 8: 424. https://doi.org/10.3390/jfb14080424
APA StyleGelli, R., & Ridi, F. (2023). An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials. Journal of Functional Biomaterials, 14(8), 424. https://doi.org/10.3390/jfb14080424