Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices
Abstract
:1. Introduction
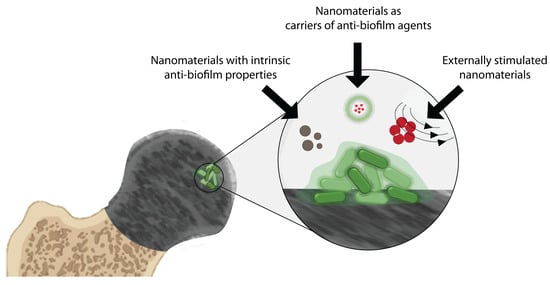
2. Nanomaterials for Treating Bacterial Biofilms on Medical Devices
2.1. Nanomaterials with Intrinsic Biofilm-Eradicating Properties
2.2. Nanomaterials as Carriers of Biofilm-Eradicating Agents
2.3. Treating Biofilms with Responsive Nanomaterials
2.3.1. Magnetically Responsive Nanomaterials
2.3.2. Local pH or Exogenous H2O2—Responsive Nanomaterials
2.3.3. Light/Heat—Responsive Nanomaterials
2.3.4. Nanomaterials with Combination of Light and Environmental pH Stimulation
3. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tran, N.; Tran, P.A. Nanomaterial-Based Treatments for Medical Device-Associated Infections. ChemPhysChem 2012, 13, 2481–2494. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Gnanadhas, D.P.; Elango, M.; Janardhanraj, S.; Srinandan, C.S.; Datey, A.; Strugnell, R.A.; Gopalan, J.; Chakravortty, D. Successful treatment of biofilm infections using shock waves combined with antibiotic therapy. Sci. Rep. 2015, 5, 17440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. When nanoparticles meet biofilms—Interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol. 2015, 6, 591. [Google Scholar] [CrossRef]
- Wang, L.-S.; Gupta, A.; Rotello, V.M. Nanomaterials for the Treatment of Bacterial Biofilms. ACS Infect. Dis. 2016, 2, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Davies, D.G.; Marques, C.N.H. A Fatty Acid Messenger Is Responsible for Inducing Dispersion in Microbial Biofilms. J. Bacteriol. 2009, 191, 1393. [Google Scholar] [CrossRef] [Green Version]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-Activated Nanoparticles for Controlled Topical Delivery of Farnesol To Disrupt Oral Biofilm Virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef] [Green Version]
- Estellés, A.; Woischnig, A.-K.; Liu, K.; Stephenson, R.; Lomongsod, E.; Nguyen, D.; Zhang, J.; Heidecker, M.; Yang, Y.; Simon, R.J.; et al. A high-affinity native human antibody disrupts biofilm from Staphylococcus aureus bacteria and potentiates antibiotic efficacy in a mouse implant infection model. Antimicrob. Agents Chemother. 2016, 60, 2292. [Google Scholar] [CrossRef] [Green Version]
- Watters, C.M.; Burton, T.; Kirui, D.K.; Millenbaugh, N.J. Enzymatic degradation of in vitro Staphylococcus aureus biofilms supplemented with human plasma. Infect. Drug Resist. 2016, 9, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Hogan, S.; Zapotoczna, M.; Stevens, N.T.; Humphreys, H.; O’Gara, J.P.; O’Neill, E. Potential use of targeted enzymatic agents in the treatment of Staphylococcus aureus biofilm-related infections. J. Hosp. Infect. 2017, 96, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Mizan, M.F.R.; Jahid, I.K.; Ha, S.-D. Microbial biofilms in seafood: A food-hygiene challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, E.P.; Gross, P.A.; Barrett, T.L.; Krause, P.J.; Martone, W.J.; McGowan, J.E., Jr.; Sweet, R.L.; Wenzel, R.P. Quality Standard for Antimicrobial Prophylaxis in Surgical Procedures. Clin. Infect. Dis. 1994, 18, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Infect. Control Hosp. Epidemiol. 1999, 20, 247–280. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef] [PubMed]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-Loaded Bone Cement for Infection Prophylaxis in Total Joint Replacement. JBJS 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Johnson, B.; Starks, I.; Bancroft, G.; Roberts, P.J. The effect of care bundle development on surgical site infection after hemiarthroplasty: An 8-year review. J. Trauma Acute Care Surg. 2012, 72, 1375–1379. [Google Scholar] [CrossRef]
- Ramos, G.; Cornistein, W.; Cerino, G.T.; Nacif, G. Systemic antimicrobial prophylaxis in burn patients: Systematic review. J. Hosp. Infect. 2017, 97, 105–114. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Tenke, P.; Riedl, C.R.; Jones, G.L.; Williams, G.J.; Stickler, D.; Nagy, E. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int. J. Antimicrob. Agents 2004, 23, 67–74. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.; Tsuchiya, H.; Morelli, I.; Battaglia, A.; Drago, L. Antibacterial coating of implants: Are we missing something? Bone Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D.; Team, I. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qasim, S.N.; Swann, A.; Ashford, R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement—A literature review. SICOT J. 2017, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.; Hwang, J.; Lee, E.; Kim, Y.J.; Lee, K.; Park, C.; Choi, Y.; Jeon, H.; Choi, J. Engineering copper nanoparticles synthesized on the surface of carbon nanotubes for anti-microbial and anti-biofilm applications. Nanoscale 2018, 10, 15529–15544. [Google Scholar] [CrossRef] [PubMed]
- Chendong, H.; Nicholas, R.; Stephen, F.; Julia, D.; Aaron, B.; Amber, L.D. Recent developments in the use of nanoparticles for treatment of biofilms. Nanotechnol. Rev. 2017, 6, 383–404. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiebner, D.W.; Barros, C.; Quinn, L.; Vitale, S.; Casey, E. Surface functionalization-dependent localization and affinity of SiO2 nanoparticles within the biofilm EPS matrix. Biofilm 2020, 2, 100029. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. biofilms: A battle against another paradigm of antibiotic resistance. MedChemComm 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Salunke, G.R.; Ghosh, S.; Santosh Kumar, R.J.; Khade, S.; Vashisth, P.; Kale, T.; Chopade, S.; Pruthi, V.; Kundu, G.; Bellare, J.R.; et al. Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int. J. Nanomed. 2014, 9, 2635–2653. [Google Scholar] [CrossRef] [Green Version]
- Gaidhani, S.; Singh, R.; Singh, D.; Patel, U.; Shevade, K.; Yeshvekar, R.; Ananda Chopade, B. Biofilm disruption activity of silver nanoparticles synthesized by Acinetobacter calcoaceticus PUCM 1005. Mater. Lett. 2013, 108, 324–327. [Google Scholar] [CrossRef]
- Habimana, O.; Zanoni, M.; Vitale, S.; O’Neill, T.; Scholz, D.; Xu, B.; Casey, E. One particle, two targets: A combined action of functionalised gold nanoparticles, against Pseudomonas fluorescens biofilms. J. Colloid Interface Sci. 2018, 526, 419–428. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic Effect of Newly Introduced Root Canal Medicaments; Ozonated Olive Oil and Chitosan Nanoparticles, Against Persistent Endodontic Pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Zhilong, S.; Gee, N.K.; Kishen, A. Nanoparticulates for Antibiofilm Treatment and Effect of Aging on Its Antibacterial Activity. J. Endod. 2010, 36, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-Y.; Lu, B.-Y.; Zhang, T.-X.; Zhang, T.; Zhang, C.-L.; Li, Y.; Peng, Q. Antibiofilm effect of drug-free and cationic poly(D,L-lactide-co-glycolide) nanoparticles via nano–bacteria interactions. Nanomedicine 2018, 13, 1093–1106. [Google Scholar] [CrossRef]
- Harper, R.A.; Carpenter, G.H.; Proctor, G.B.; Harvey, R.D.; Gambogi, R.J.; Geonnotti, A.R.; Hider, R.; Jones, S.A. Diminishing biofilm resistance to antimicrobial nanomaterials through electrolyte screening of electrostatic interactions. Colloids Surf. B Biointerfaces 2019, 173, 392–399. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [Green Version]
- Rukavina, Z.; Vanić, Ž. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics 2016, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Wolfmeier, H.; Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. New Perspectives in Biofilm Eradication. ACS Infect. Dis. 2018, 4, 93–106. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine 2014, 10, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr. Polym. 2018, 200, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Elbi, S.; Nimal, T.R.; Rajan, V.K.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of Salmonella. Colloids Surf. B Biointerfaces 2017, 160, 40–47. [Google Scholar] [CrossRef]
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria. Sci. Rep. 2016, 6, 18877. [Google Scholar] [CrossRef] [Green Version]
- Baelo, A.; Levato, R.; Julián, E.; Crespo, A.; Astola, J.; Gavaldà, J.; Engel, E.; Mateos-Timoneda, M.A.; Torrents, E. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control. Release 2015, 209, 150–158. [Google Scholar] [CrossRef]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.-S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-Stabilized Capsules for the Treatment of Bacterial Biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef] [Green Version]
- Landis, R.F.; Gupta, A.; Lee, Y.-W.; Wang, L.-S.; Golba, B.; Couillaud, B.; Ridolfo, R.; Das, R.; Rotello, V.M. Cross-Linked Polymer-Stabilized Nanocomposites for the Treatment of Bacterial Biofilms. ACS Nano 2017, 11, 946–952. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of Size and Shape on Biofilm Eradication for Nitric Oxide-Releasing Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329. [Google Scholar] [CrossRef]
- Mihu, M.R.; Cabral, V.; Pattabhi, R.; Tar, M.T.; Davies, K.P.; Friedman, A.J.; Martinez, L.R.; Nosanchuk, J.D. Sustained Nitric Oxide-Releasing Nanoparticles Interfere with Methicillin-Resistant Staphylococcus aureus Adhesion and Biofilm Formation in a Rat Central Venous Catheter Model. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Cheeseman, S.; Christofferson, A.J.; Kariuki, R.; Cozzolino, D.; Daeneke, T.; Crawford, R.J.; Truong, V.K.; Chapman, J.; Elbourne, A. Antimicrobial Metal Nanomaterials: From Passive to Stimuli-Activated Applications. Adv. Sci. 2020, 7, 1902913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, M.K.; Biswas, R.; Biswas, L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019, 309, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Taylor, E.N.; Kummer, K.M.; Durmus, N.G.; Leuba, K.; Tarquinio, K.M.; Webster, T.J. Superparamagnetic iron oxide nanoparticles (SPION) for the treatment of antibiotic-resistant biofilms. Small 2012, 8, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Leuba, K.D.; Durmus, N.G.; Taylor, E.N.; Webster, T.J. Short communication: Carboxylate functionalized superparamagnetic iron oxide nanoparticles (SPION) for the reduction of S. aureus growth post biofilm formation. Int. J. Nanomed. 2013, 8, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Du, C.; Liao, J.-Y.; Gu, Y.; Gong, Y.; Pei, J.; Gu, H.; Yin, D.; Gao, L.; Pan, Y. Synthesis of magnetite hybrid nanocomplexes to eliminate bacteria and enhance biofilm disruption. Biomater. Sci. 2019, 7, 2833–2840. [Google Scholar] [CrossRef]
- Gao, L.; Giglio, K.M.; Nelson, J.L.; Sondermann, H.; Travis, A.J. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale 2014, 6, 2588–2593. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Teirlinck, E.; Xiong, R.; Brans, T.; Forier, K.; Fraire, J.; Van Acker, H.; Matthijs, N.; De Rycke, R.; De Smedt, S.C.; Coenye, T.; et al. Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms. Nat. Commun. 2018, 9, 4518. [Google Scholar] [CrossRef]
- Teirlinck, E.; Barras, A.; Liu, J.; Fraire, J.C.; Lajunen, T.; Xiong, R.; Forier, K.; Li, C.; Urtti, A.; Boukherroub, R.; et al. Exploring Light-Sensitive Nanocarriers for Simultaneous Triggered Antibiotic Release and Disruption of Biofilms Upon Generation of Laser-Induced Vapor Nanobubbles. Pharmaceutics 2019, 11, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, W.; Chen, Z.; Sun, H.; Shi, P.; Gao, N.; Ren, J.; Qu, X. Visible-light-driven enhanced antibacterial and biofilm elimination activity of graphitic carbon nitride by embedded Ag nanoparticles. Nano Res. 2015, 8, 1648–1658. [Google Scholar] [CrossRef]
- Hu, D.; Li, H.; Wang, B.; Ye, Z.; Lei, W.; Jia, F.; Jin, Q.; Ren, K.-F.; Ji, J. Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus aureus Biofilm. ACS Nano 2017, 11, 9330–9339. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Sun, P.; Zhang, L.; Liu, Z.; Wang, H.; Ren, J.; Qu, X. Porphyrin MOF Dots–Based, Function-Adaptive Nanoplatform for Enhanced Penetration and Photodynamic Eradication of Bacterial Biofilms. Adv. Funct. Mater. 2019, 29, 1903018. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Bragonzi, A.; Boletta, A.; Biffi, A.; Muggia, A.; Sersale, G.; Cheng, S.H.; Bordignon, C.; Assael, B.M.; Conese, M. Comparison between cationic polymers and lipids in mediating systemic gene delivery to the lungs. Gene Ther. 1999, 6, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. Biomed. Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villiers, C.; Freitas, H.; Couderc, R.; Villiers, M.-B.; Marche, P. Analysis of the toxicity of gold nano particles on the immune system: Effect on dendritic cell functions. J. Nanopart. Res. 2010, 12, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Gristina, A.G.; Naylor P Fau-Myrvik, Q.; Myrvik, Q. Infections from biomaterials and implants: A race for the surface. Med. Prog. Technol. 1988, 14, 205. [Google Scholar] [PubMed]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.A.; Ly, K.L.; Fox, K.E.; Tran, P.A.; Nguyen, T.H. Immobilization of Antimicrobial Silver and Antioxidant Flavonoid as a Coating for Wound Dressing Materials. Int. J. Nanomed. 2019, 14, 9929–9939. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.A.; Tran, P.A. Immobilization-Enhanced Eradication of Bacterial Biofilms and in situ Antimicrobial Coating of Implant Material Surface—An in vitro Study. Int. J. Nanomed. 2019, 14, 9351–9360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Liu, X.; Xu, Z.; Yeung, K.W.K.; Wu, S. Dopamine Modified Organic–Inorganic Hybrid Coating for Antimicrobial and Osteogenesis. ACS Appl. Mater. Interfaces 2016, 8, 33972–33981. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182. [Google Scholar] [CrossRef] [Green Version]
- Swartjes, J.J.T.M.; Das, T.; Sharifi, S.; Subbiahdoss, G.; Sharma, P.K.; Krom, B.P.; Busscher, H.J.; van der Mei, H.C. A Functional DNase I Coating to Prevent Adhesion of Bacteria and the Formation of Biofilm. Adv. Funct. Mater. 2013, 23, 2843–2849. [Google Scholar] [CrossRef]
- Shi, L.; Santhanakrishnan, S.; Cheah, Y.S.; Li, M.; Chai, C.L.L.; Neoh, K.G. One-Pot UV-Triggered o-Nitrobenzyl Dopamine Polymerization and Coating for Surface Antibacterial Application. ACS Appl. Mater. Interfaces 2016, 8, 33131–33138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.; Thappeta, K.R.V.; Subramanian, J.T.L.; Pranantyo, D.; Kang, E.-T.; Duan, H.; Kline, K.; Chan-Park, M.B. In Vivo Anti-Biofilm and Anti-Bacterial Non-Leachable Coating Thermally Polymerized on Cylindrical Catheter. ACS Appl. Mater. Interfaces 2017, 9, 36269–36280. [Google Scholar] [CrossRef] [PubMed]
- Adnan, N.N.M.; Sadrearhami, Z.; Bagheri, A.; Nguyen, T.-K.; Wong, E.H.H.; Ho, K.K.K.; Lim, M.; Kumar, N.; Boyer, C. Exploiting the Versatility of Polydopamine-Coated Nanoparticles to Deliver Nitric Oxide and Combat Bacterial Biofilm. Macromol. Rapid Commun. 2018, 39, 1800159. [Google Scholar] [CrossRef] [PubMed]








| Activation Mechanism | Materials | Size and Shape | Preformed Bacterial Biofilm | Treatment Duration | Antibiofilm Efficacy | Ref. | |
|---|---|---|---|---|---|---|---|
| EPS Disruption | Bacterial Killing | ||||||
| Intrinsic | Biosynthesized AgNPs from P. zeylanica | spheres 15–295 nm | 24 h-A. baumannii, E. coli, and S. aureus 24 h-mixed species biofilm (A. baumannii–S. aureus) | 24 h | 88%, 67%, 78%, 64% | 96% to 99% | [38] |
| biosynthesized AgNPs from Acinetobacter calcoaceticus | spheres 4–40 nm | 24 h-20 different pathogen bacterial biofilm | 24 h | Up to 98% | NR | [39] | |
| AuNPs + proteinase K | spheres 11–27 nm | 72 h-Pseudomonas fluorescens biofilm | 24 h | 74% | 50% | [40] | |
| Chitosan NPs + O3 oil | NR | 24 h-mixed-species biofilm (S. mutans, E. faecalis, and C. albicans) | 8 days | NR | 62.5% | [44] | |
| Chitosan NPs | spheres 220 ± 3 nm | 7-day-E. faecalis biofilm | 72 h | 44% | 75–80% | [45] | |
| PLGA + Octadecylamide NPs | spheres 217.7 nm | 24 h-S. mutants biofilm | 72 h | NR | Up to 73% | [46] | |
| α-TP + Phosphate α-TP + Trizma | 700 nm | 18 h-multispecies oral biofilms | 2 min | NR | ~45% | [47] | |
| Nanocarriers | chitosan + DNase I + oxacillin | Spheres 166.7 nm | 24 h-S. aureus biofilm | 24 h | Up to 100% | [53] | |
| Chitosan NPs + ciprofloxacin (Cip)+ Fucoidan | spheres 235 ± 25 nm | 48 h-S. Paratyphi A biofilm | 48 h | 62% in vitro 95% in vivo | [54] | ||
| Gentamicin + phosphatidylcholine + AuNPs | Spheres ~180 nm | 24 h-P. aeruginosa biofilm 24 h-S. aureus biofilm 24 h-E. coli biofilm 24 h-L. monocytogenes biofilm | 24 h | 68.75% 66.67% 69.23% 65.38% | [55] | ||
| PLGA -Poly(lysin) + DNase I + Cip | spheres 251.9 nm | 48 h-P. aeruginosa biofilm 24 h-P. aeruginosa biofilm | 24 h | NR | 43% 95% | [56] | |
| Silica + cinnamaldehyde NPs-peppermint oil capsule | NPs: ~150 nm Capsules: 6.7 ± 1.9 μm | 24 h-E. coli DH5, P. aeruginosa, S. aureus, and E. cloacae | 3 h | NR | 90–100% | [57] | |
| copolymer Poly(oxanorborneneimide) bearing guanidine, amine, and tetraethylene glycol monomethyl ether units + poly(maleic anhydride-alt-octadecene + carvacrol oil | Spheres: ~250 nm | 24 h-E. coli DH5, P. aeruginosa, S. aureus, and E. cloacae | 3 h | NR | 90–95% | [58] | |
| Silica NPs + nitric oxide (NO) | Spheres 14–150 nm | 48 h-S. aureus and P. aeruginosa biofilm | 24 h | NR | 75% | [59] | |
| polyethylene glycol + glucose + chitosan + sodium nitrite + NO | spheres 10 nm | 24 h-MRSA biofilm | 24 h | NR | 75% | [60] | |
| Magnetically responsive | iron oxide (IONs) + Zn IONs + Ag IONs + Fe | 19.67 ± 0.72 nm 193.72 ± 6.15 nm 23.32 ± 1.17 nm | 24 h-S. aureus biofilm | 24 h | 85%, 40%, 50% | [64] | |
| IONs + amine IONs + carboxylate IONs + isocyanate | Spheres 14–19 nm | 24 h-old S. aureus biofilm | 24 h | NR | 28.1%, 33.5% 31.1% | [65] | |
| silver + iron oxide + magnetic | Spheres 45–55 nm | 24 h-E. coli biofilm 24 h-P. aeruginosa biofilm | 88% 90% | [66] | |||
| Local pH or exogenous H2O2 | Fe3O4 + H2O2 | spheres 500 nm | Overnight P. aeruginosa biofilm | 2 h | 82% | [67] | |
| IONs + H2O2 | Spheres 213 ± 26 nm | 19 h-S. mutants in vitro | 5 or 10 min, twice daily, 43 h | 57–60% | 99.9% | [70] | |
| DMAEMA + BMA + PAA + Farnesol | Sphere ∼60 nm | 24 h-S. mutants biofilms | 44 h | 50% | 80% | [8] | |
| Light/heat | Nanobubbles produced by AuNPs + tobramycin | 70 nm | 24 h-B. multivorans biofilm 24 h-P. aeruginosa biofilm 24 h-S. aureus biofilm | 3 min laser and 24 h tobramycin | NR | 98% 95% 96% | [71] |
| phospholipid 1,2-dioleoyl-sn-glycero-3-phosphocholine+ phospholipid 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) AuNPs + tobramycin graphene quantum dot + AuNPs + tobramycin | 182 ± 102 nm 39 ± 14 nm | 24 h-P. aeruginosa biofilm | 3 min laser and 24 h tobramycin | 75% | [72] | ||
| 15 min laser exposure and 24 h tobramycin | 62% | ||||||
| Ag/g-C3N4 | g-C3N4 Nanosheet AgNPs: 6.5 nm | 48 h-S. aureus biofilm | 3 h | 70% | 100% | [73] | |
| Chitosan NPs + Rose Bengal | spheres 60 ± 20 nm | 21-day-biofilm | 15 min irradiation from Lumacare lamp at 540 ± 15 nm | 40–60% | [52] | ||
| Light and environmental pH | Citrate-caped AuNPs | spheres 14 nm | 24 h-MRSA biofilm in vitro 24 h-subcutaneous abscess | 60 min 10 min NIR light irradiation for 7 days | 92% 88% | [74] | |
| pMOF nanodots + porphyrin + MnO2 + human serum albumin + H2O2 | Spheres 105 nm | 48 h-S. aureus biofilm 2-day-subcutaneous abscess | 15 min visible light irradiation | 87.5% 99.9% | [75] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, H.M.; Tran, H.; Booth, M.A.; Fox, K.E.; Nguyen, T.H.; Tran, N.; Tran, P.A. Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices. Nanomaterials 2020, 10, 2253. https://doi.org/10.3390/nano10112253
Tran HM, Tran H, Booth MA, Fox KE, Nguyen TH, Tran N, Tran PA. Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices. Nanomaterials. 2020; 10(11):2253. https://doi.org/10.3390/nano10112253
Chicago/Turabian StyleTran, Hoai My, Hien Tran, Marsilea A. Booth, Kate E. Fox, Thi Hiep Nguyen, Nhiem Tran, and Phong A. Tran. 2020. "Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices" Nanomaterials 10, no. 11: 2253. https://doi.org/10.3390/nano10112253
APA StyleTran, H. M., Tran, H., Booth, M. A., Fox, K. E., Nguyen, T. H., Tran, N., & Tran, P. A. (2020). Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices. Nanomaterials, 10(11), 2253. https://doi.org/10.3390/nano10112253