Optimized Preparation of Nanosized Hollow SSZ-13 Molecular Sieves with Ultrasonic Assistance
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of SSZ-13
2.2. Characterization
3. Results and Discussion
3.1. Effect of the Amount of Seed on the Sample Structure
3.2. Effect of the Ultrasonic Time on the Sample Structure
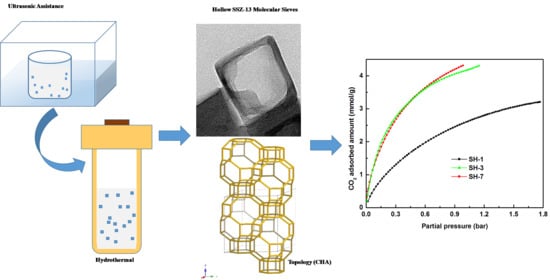
3.3. CO2 Adsorption Performance of Hollow SSZ-13
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Gu, Q.; Hanif, A.; Wang, T.; Shang, J. Transition metal cation-exchanged SSZ-13 zeolites for CO2 capture andseparation from N2. Chem. Eng. J. 2019, 370, 1450–1458. [Google Scholar] [CrossRef]
- Shang, J.; Li, G.; Singh, R.; Gu, Q.; Nairn, K.M.; Bastow, T.J.; Medhekar, N.; Doherty, C.M.; Hill, A.J.; Liu, J.Z.; et al. Discriminative Separation of Gases by a “Molecular Trapdoor” Mechanism in Chabazite Zeolites. J. Am. Chem. Soc. 2012, 134, 19246–19253. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zhou, L.; Luo, Y. Controllable synthesis of Si-DD3R molecular sieves nanocrystallineby microwave assisting dry-gel conversion method. Mater. Res. Express 2020, 7, 085014. [Google Scholar] [CrossRef]
- Jiang, Q.; Rentschler, J.; Sethia, G.; Weinman, S.; Perrone, R.; Liu, K. Synthesis of T-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 byadsorption process. Chem. Eng. J. 2013, 230, 380–388. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, J.; Wang, J.; Wang, L.; Xu, C.; Shen, M. Efficient Hydrothermal Synthesis of SSZ-13 with Variable Grain Size. Materials 2020, 13, 1829. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Guo, Q.; Bian, H.; Lan, A.; Li, X.; Han, P.; Dou, T. Efficient synthesis of high silica SSZ-13 zeolite via a steam-assisted crystallization process. J. Porous Mater. 2019, 26, 1879–1888. [Google Scholar] [CrossRef]
- Niu, K.; Li, G.; Liu, J.; Wei, Y. One step synthesis of Fe-SSZ-13 zeolite by hydrothermal method. J. Solid State Chem. 2020, 287, 121330. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stability. Appl. Catal. B Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Lv, Y.; Ye, C.; Zhang, J.; Guo, C. Rapid and efficient synthesis of highly crystalline SSZ-13 zeolite by applying high shear mixing in the aging process. Microporous Mesoporous Mater. 2020, 293, 109812. [Google Scholar] [CrossRef]
- Liu, Z.; Wakihara, T.; Oshima, K.; Nishioka, D.; Hotta, Y.; Elangovan, S.P.; Yanaba, Y.; Yoshikawa, T.; Chaikittisilp, W.; Matsuo, T.; et al. Widening Synthesis Bottlenecks: Realization of Ultrafast and Continuous-Flow Synthesis of High-Silica Zeolite SSZ-13 for NOx Removal. Angew. Chem. 2015, 127, 5775–5779. [Google Scholar] [CrossRef]
- Chen, B.; Xu, R.; Zhang, R.; Liu, N. Economical Way to Synthesize SSZ-13 with Abundant Ion-Exchanged Cu+ for an Extraordinary Performance in Selective Catalytic Reduction (SCR) of NOx by Ammonia. Environ. Sci. Technol. 2014, 48, 13909–13916. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zhang, Y.; Xie, Y. Application of Mn3O4 nanowires in the dye waste water treatment at room temperature. Sep. Purif. Technol. 2020, 234, 116119. [Google Scholar] [CrossRef]
- Han, R.; Chen, M.; Liu, X.-B.; Zhang, Y.; Xie, Y.; Sui, Y. Controllable Synthesis of Mn3O4 Nanowires and Application in the Treatment of Phenol at Room Temperature. Nanomaterials 2020, 10, 461. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, D.; Chu, W.; Yang, C.; Wang, Y.; Zhu, X.; Xin, W.; Liu, Z.; Wang, H.; Liu, S.; et al. N-methyl-2-pyrrolidone-induced conversion of USY into hollow Beta zeolite and its application in the alkylation of benzene with isobutylene. Microporous Mesoporous Mater. 2020, 294, 109944. [Google Scholar] [CrossRef]
- Wang, J.; Wan, J.; Yang, N.; Li, Q.; Wang, D. Hollow multishell structures exercise temporal–spatial ordering and dynamic smart behaviour. Nat. Rev. Chem. 2020, 4, 159–168. [Google Scholar] [CrossRef]
- Chu, N.; Wang, J.; Zhang, Y.; Yang, J.; Lu, J.; Yin, D. Nestlike Hollow Hierarchical MCM-22 Microspheres: Synthesis and Exceptional Catalytic Properties. Chem. Mater. 2010, 22, 2757–2763. [Google Scholar] [CrossRef]
- Jia, X.; Fan, H.; Zhang, F.; Qin, L. Using sonochemistry for the fabrication of hollow ZnO microspheres. Ultrason. Sonochem. 2010, 17, 284–287. [Google Scholar] [CrossRef]
- Hamidzadeh, M.; Saeidi, M.; Komeili, S. Modified seeding method to produce hierarchical nanocrystalline ZSM-5 zeolite. Mater. Today Commun. 2020, 25, 101308. [Google Scholar] [CrossRef]
- Zarekarizi, F.; Morsali, A. Ultrasonic-assisted synthesis of nano-sized metal-organic framework; a simple method to explore selective and fast Congo Red adsorption. Ultrason. Sonochem. 2020, 69, 105246. [Google Scholar] [CrossRef]
- Deng, C.; Hu, H.; Ge, X.; Han, C.; Zhao, D.; Shao, G. One-pot sonochemical fabrication of hierarchical hollow CuOsubmicrospheres. Ultrason. Sonochem. 2011, 18, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Ehsani, A.; Rostami-Vartouni, A. Ultrasound-promoted green approach for the synthesis of sulfonamides using natural, stable and reusable Natrolitenanozeolite catalyst at room temperature. Ultrason. Sonochem. 2014, 21, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Behin, J.; Kazemian, H.; Rohani, S. Sonochemical synthesis of zeolite NaP from clinoptilolite. Ultrason. Sonochem. 2016, 28, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.R.; Queen, W.L.; Mason, J.A.; Fickel, D.W.; Lobo, R.F.; Brown, C.M. Unconventional, Highly Selective CO2 Adsorption in Zeolite SSZ-13. J. Am. Chem. Soc. 2012, 134, 1970–1973. [Google Scholar] [CrossRef] [PubMed]











| Sample | Solution Composition SDA:NaOH:Al:Si:H2O (wt.% Seed) | Al Source | T/°C | t/h | Ultrasonic Time/Min |
|---|---|---|---|---|---|
| SH-1 | 20:20:5:100:4400 (0) | Al (OH)3 | 160 | 96 | 15 |
| SH-2 | 20:20:5:100:4400 (0.25) | Al (OH)3 | 160 | 96 | 15 |
| SH-3 | 20:20:5:100:4400:0 (0.5) | Al (OH)3 | 160 | 96 | 15 |
| SH-4 | 20:20:5:100:4400:0 (1) | Al (OH)3 | 160 | 96 | 15 |
| SH-5 | 20:20:5:100:4400:0 (0.5) | Al (OH)3 | 160 | 96 | 30 |
| SH-6 | 20:20:5:100:4400:0 (0.5) | Al (OH)3 | 160 | 96 | 45 |
| SH-7 | 20:20:5:100:4400:0 (0.5) | Al (OH)3 | 160 | 96 | 60 |
| Sample | SBET (m2/g) | Total Pore Volume 1,3 (cm3/g) | Mesoporous Volume 2 (cm3/g) | Micropore Volume 1 (cm3/g) | Average Pore Width (nm) | Content of Mesoporous (%) |
|---|---|---|---|---|---|---|
| SH-1 | 574.91 | 0.29 | 0.04 | 0.25 | 6.393 | 13.8 |
| SH-2 | 671.67 | 0.57 | 0.28 | 0.28 | 16.74 | 49.1 |
| SH-3 | 610.43 | 0.60 | 0.34 | 0.28 | 18.50 | 56.7 |
| SH-4 | 622.96 | 0.60 | 0.32 | 0.29 | 22.44 | 53.3 |
| Sample | SBET (m2/g) | Total Pore Volume 1,3 (cm3/g) | Mesoporous Volume 2 (cm3/g) | Micropore Volume 1 (cm3/g) | Average Pore Width (nm) | Content of Mesoporous (%) |
|---|---|---|---|---|---|---|
| SH-5 | 653.45 | 0.68 | 0.41 | 0.26 | 21.53 | 60.3 |
| SH-6 | 763.36 | 0.78 | 0.47 | 0.30 | 19.11 | 60.3 |
| SH-7 | 791.50 | 0.95 | 0.63 | 0.31 | 21.96 | 66.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Han, R.; Tao, Y.; Wang, J.; Luo, Y. Optimized Preparation of Nanosized Hollow SSZ-13 Molecular Sieves with Ultrasonic Assistance. Nanomaterials 2020, 10, 2298. https://doi.org/10.3390/nano10112298
Zhou L, Han R, Tao Y, Wang J, Luo Y. Optimized Preparation of Nanosized Hollow SSZ-13 Molecular Sieves with Ultrasonic Assistance. Nanomaterials. 2020; 10(11):2298. https://doi.org/10.3390/nano10112298
Chicago/Turabian StyleZhou, Liang, Runlin Han, Yuxuan Tao, Jinqu Wang, and Yiwei Luo. 2020. "Optimized Preparation of Nanosized Hollow SSZ-13 Molecular Sieves with Ultrasonic Assistance" Nanomaterials 10, no. 11: 2298. https://doi.org/10.3390/nano10112298
APA StyleZhou, L., Han, R., Tao, Y., Wang, J., & Luo, Y. (2020). Optimized Preparation of Nanosized Hollow SSZ-13 Molecular Sieves with Ultrasonic Assistance. Nanomaterials, 10(11), 2298. https://doi.org/10.3390/nano10112298