Microwave-Assisted Synthesis of Chalcopyrite/Silver Phosphate Composites with Enhanced Degradation of Rhodamine B under Photo-Fenton Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
2.2. Characterization of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
2.3. Degradation Procedure by Using Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
2.4. Evaluation of Charge Separation and Recombination Rate of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
2.5. Free Radical Trapping Experiment of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
3. Results and Discussion
3.1. Morphology and Crystal Phase of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
3.2. Optical Property of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
3.3. Degradation Performance of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
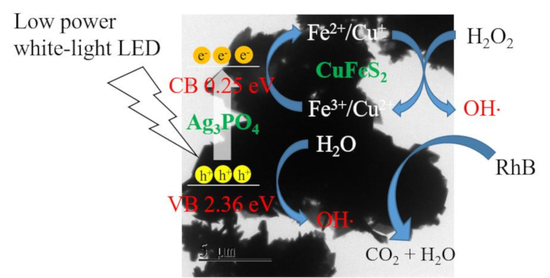
3.4. Degradation Mechanism of Ag3PO4, CuFeS2, and CuFeS2/Ag3PO4
3.5. Stability and Practical Applications of CuFeS2/Ag3PO4
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fernandez-Llamazares, A.; Garteizgogeascoa, M.; Basu, N.; Brondizio, E.S.; Cabeza, M.; Martinez-Alier, J.; McElwee, P.; Reyes-Garcia, V. A state-of-the-art review of indigenous peoples and environmental pollution. Integr. Environ. Assess. Manag. 2020, 16, 324–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurwadkar, S. Occurrence and distribution of organic and inorganic pollutants in groundwater. Water Environ. Res. 2019, 91, 1001–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Muszynski, P.; Brodowska, M.S.; Paszko, T. Occurrence and transformation of phenoxy acids in aquatic environment and photochemical methods of their removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 1276–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghipour, S.; Hosseini, S.M.; Ataie-Ashtiani, B. Engineering nanomaterials for water and wastewater treatment: Review of classifications, properties and applications. New J. Chem. 2019, 43, 7902–7927. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean Prod. 2018, 196, 1669–1680. [Google Scholar] [CrossRef]
- Wang, J.M.; Shih, Y.E.; Wang, P.Y.; Yu, Y.H.; Su, J.F.; Huang, C.P. Hazardous waste treatment technologies. Water Environ. Res. 2019, 91, 1177–1198. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. Sci. Eng. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.X.; Zhao, W.; Zhang, T.; Zhan, Y.J.; Xie, R.J.; Leung, D.Y.C.; Huang, H.B. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Morales, M.A.; Fernandez-Cervantes, I.; Agustin-Serrano, R.; Ruiz-Salgado, S.; Sampedro, M.P.; Varela-Caselis, J.L.; Portillo, R.; Rubio, E. Ag3PO4 microcrystals with complex polyhedral morphologies diversity obtained by microwave-hydrothermal synthesis for MB degradation under sunlight. Results Phys. 2019, 12, 1344–1356. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Liu, Q.W.; Licao, Y.Q.; Liu, G.D.; Shi, X.H. Z-scheme AgSCN/Ag3PO4/C3N4 heterojunction with excellent photocatalytic degradation of ibuprofen. Ceram. Int. 2020, 46, 106–113. [Google Scholar] [CrossRef]
- Ge, M.; Li, Z.L. Recent progress in Ag3PO4-based all-solid-state Z-scheme photocatalytic systems. Chin. J. Catal. 2017, 38, 1794–1803. [Google Scholar] [CrossRef]
- Bi, Z.H.; Li, Z.H.; Yan, L.F. Catalytic oxidation of lignin to dicarboxylic acid over the CuFeS2 nanoparticle catalyst. Green Process. Synth. 2018, 7, 306–315. [Google Scholar] [CrossRef]
- Labiadh, L.; Ammar, S.; Karnali, A.R. Oxidation/mineralization of AO7 by electro-Fenton process using chalcopyrite as the heterogeneous source of iron and copper catalysts with enhanced degradation activity and reusability. J. Electroanal. Chem. 2019, 853, 113532. [Google Scholar] [CrossRef]
- Ltaief, A.H.; Pastrana-Martinez, L.M.; Ammar, S.; Gadri, A.; Faria, J.L.; Silva, A.M.T. Mined pyrite and chalcopyrite as catalysts for spontaneous acidic pH adjustment in Fenton and LED photo-Fenton-like processes. J. Chem. Technol. Biotechnol. 2018, 93, 1137–1146. [Google Scholar] [CrossRef]
- Nie, W.S.; Mao, Q.H.; Ding, Y.B.; Hu, Y.; Tang, H.Q. Highly efficient catalysis of chalcopyrite with surface bonded ferrous species for activation of peroxymonosulfate toward degradation of bisphenol A: A mechanism study. J. Hazard. Mater. 2019, 364, 59–68. [Google Scholar] [CrossRef]
- Xu, X.J.; Tang, D.D.; Cai, J.H.; Xi, B.D.; Zhang, Y.; Pi, L.; Mao, X.H. Heterogeneous activation of peroxymonocarbonate by chalcopyrite (CuFeS2) for efficient degradation of 2,4-dichlorophenol in simulated groundwater. Appl. Catal. B Environ. 2019, 251, 273–282. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Zhu, R.L.; Xi, Y.F.; Zhu, J.X.; Zhu, G.Q.; He, H.P. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Salla, J.D.; Martinello, K.D.; Dotto, G.L.; Garcia-Diaz, E.; Javed, H.; Alvarez, P.J.J.; Foletto, E.L. Synthesis of citrate-modified CuFeS2 catalyst with significant effect on the photo-Fenton degradation efficiency of bisphenol a under visible light and near-neutral pH. Colloid Surf. A Physicochem. Eng. Asp. 2020, 595, 124679. [Google Scholar] [CrossRef]
- Zhang, J.B.; Sun, X.H.; Gao, Q.J.; Wang, H.X.; Liang, D.X.; Liu, Z.M.; Han, G.T.; Jiang, W. Degradation of organic dyes over regenerative Fe3O4/CuFeS2/biomass composite column. Chem. J. Chin. Univ. Chin. 2019, 40, 425–430. [Google Scholar]
- Wei, T.T.; Wu, T.; Lin, Y.W. Controlled synthesis of Ag3PO4 microparticles with different morphologies and their photocatalytic degradation of rhodamine B under white light-emitting diode irradiation. Micro Nano Lett. 2019, 14, 363–366. [Google Scholar] [CrossRef]
- Rajaji, U.; Murugan, K.; Chen, S.M.; Govindasamy, M.; Chen, T.W.; Lin, P.H.; Prabha, P.L. Graphene oxide encapsulated 3D porous chalcopyrite (CuFeS2) nanocomposite as an emerging electrocatalyst for agro-hazardous (methyl paraoxon) detection in vegetables. Compos. Part B Eng. 2019, 160, 268–276. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, T.H.; Lin, Y.W. Preparation and characterization of bismuth oxychloride/reduced graphene oxide for photocatalytic degradation of rhodamine B under white-light light-emitting-diode and sunlight irradiation. J. Photochem. Photobiol. A Chem. 2019, 371, 355–364. [Google Scholar] [CrossRef]
- Huang, C.W.; Wu, M.Y.; Lin, Y.W. Solvothermal synthesis of Ag hybrid BiPO4 heterostructures with enhanced photodegradation activity and stability. J. Colloid Interface Sci. 2017, 490, 217–225. [Google Scholar] [CrossRef]
- Huang, C.K.; Wu, T.; Huang, C.W.; Lai, C.Y.; Wu, M.Y.; Lin, Y.W. Enhanced photocatalytic performance of BiVO4 in aqueous AgNO3 solution under visible light irradiation. Appl. Surf. Sci. 2017, 399, 10–19. [Google Scholar] [CrossRef]
- Hu, X.F.; Mohamood, T.; Ma, W.H.; Chen, C.C.; Zhao, J.C. Oxidative decomposition of rhodamine B dye in the presence of VO2+ and/or Pt(IV) under visible light irradiation: N-deethylation, chromophore cleavage, and mineralization. J. Phys. Chem. B 2006, 110, 26012–26018. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Zhao, J.C.; Shen, T.; Hidaka, H. TiO2-assisted photodegradation of dyes: A study of two competitive primary processes in the degradation of RB in an aqueous TiO2 colloidal solution. J. Mol. Catal. A Chem. 1998, 129, 257–268. [Google Scholar] [CrossRef]
- Huang, T.Y.; Chen, Y.J.; Lai, C.Y.; Lin, Y.W. Synthesis, characterization, enhanced sunlight photocatalytic properties, and stability of Ag/Ag3PO4 nanostructure-sensitized BiPO4. RSC Adv. 2015, 5, 43854–43862. [Google Scholar] [CrossRef]
- Bansal, P.; Verma, A.; Mehta, C.; Sangal, V.K. Potential use of waste foundry sand in dual process (photocatalysis and photo-Fenton) for the effective removal of phenazone from water: Slurry and fixed-bed approach. J. Environ. Manag. 2019, 233, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Verma, A. N, Ag co-doped TiO2 mediated modified in-situ dual process (modified photocatalysis and photo-Fenton) in fixed-mode for the degradation of Cephalexin under solar irradiations. Chemosphere 2018, 212, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Verma, A. Synergistic effect of dual process (photocatalysis and photo-Fenton) for the degradation of Cephalexin using TiO2 immobilized novel clay beads with waste fly ash/foundry sand. J. Photochem. Photobiol. A Chem. 2017, 342, 131–142. [Google Scholar] [CrossRef]
- Lima, M.J.; Silva, C.G.; Silva, A.M.T.; Lopes, J.C.B.; Dias, M.M.; Faria, J.L. Homogeneous and heterogeneous photo-Fenton degradation of antibiotics using an innovative static mixer photoreactor. Chem. Eng. J. 2017, 310, 342–351. [Google Scholar] [CrossRef]


















| Series | Degradation Mode | Pseudo-First-Order Kinetic Equation | k(min−1) | R2 |
|---|---|---|---|---|
| Ag3PO4 | Photocatalytic reaction | y = 0.016x + 0.048 | 0.016 | 0.96 |
| Fenton reaction | y = 0.0026x + 0.0088 | 0.0026 | 0.92 | |
| Photo-Fenton reaction | y = 0.0047x + 0.055 | 0.0047 | 0.80 | |
| CuFeS2 | Photocatalytic reaction | y = 0.0007x − 0.039 | 0.0007 | 0.72 |
| Fenton reaction | y = 1.5x − 0.16 | 1.5 | 0.93 | |
| Photo-Fenton reaction | y = 2.8x − 0.29 | 2.8 | 0.94 | |
| CuFeS2/Ag3PO4 | Photocatalytic reaction | y = 0.018x − 0.0015 | 0.018 | 0.94 |
| Fenton reaction | y = 2.2x − 0.156 | 2.2 | 0.99 | |
| Photo-Fenton reaction | y = 3.3x − 0.0322 | 3.3 | 0.97 |
| Samples | Preparation | Degradation Performance | Sunlight Irradiation | Target | Ref. |
|---|---|---|---|---|---|
| Citrate–CuFeS2 | Microwave heating | 90% degradation (0.2 g catalyst/50 ppm BPA) within 15 min (4 W fluorescent lamp) | - | BPA | [24] |
| FS–TiO2 disk | Dip-coating method | 95% degradation (50 ppm phenazone (PNZ)) within 180 min (36 W UV light) | 95% degradation of 50 ppm PNZ within 90 min | PNZ | [34] |
| Mined CuFeS2 | Milling process | 85% TOC conversion (1.0 g catalyst/0.5 mM tyrosol (TY)) within 60 min (10 W UV LED light) | - | TY | [19] |
| Fe–N–Ag–TiO2 clay bead | Surface impregnation method | - | 77% degradation of 50 ppm cephalexin (CEX) within 60 min | CEX | [35] |
| FS/FA/TiO2 clay bead | Dip-coating method | 89% degradation (50 ppm CEX) within 4 h (36 W UV light) | 94% degradation (50 ppm CEX) within 3.5 h | CEX | [36] |
| Fe2O3–TiO2 film | Sol–gel method | 80% degradation (10 ppm ciprofloxacin (CIPRO), sulfamethoxazole (SMX), and trimethoprim (TMP) mixture) within 240 min by a solar simulator (Solarbox Model 1500e) | - | CIPRO, SMX, TMP | [37] |
| CuFeS2/Ag3PO4 | Cyclic microwave heating | 96% degradation (20 mg catalyst/20 ppm RhB) within 1 min (2.5 W white-light LED) | 99.8% degradation (0.15 g catalyst/15 ppm MB) within 6 min | RhB, MR, R6G, Fluorescein, PI, phenol | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-A.; Wen, P.-Y.; Wu, T.; Lin, Y.-W. Microwave-Assisted Synthesis of Chalcopyrite/Silver Phosphate Composites with Enhanced Degradation of Rhodamine B under Photo-Fenton Process. Nanomaterials 2020, 10, 2300. https://doi.org/10.3390/nano10112300
Chang S-A, Wen P-Y, Wu T, Lin Y-W. Microwave-Assisted Synthesis of Chalcopyrite/Silver Phosphate Composites with Enhanced Degradation of Rhodamine B under Photo-Fenton Process. Nanomaterials. 2020; 10(11):2300. https://doi.org/10.3390/nano10112300
Chicago/Turabian StyleChang, Shun-An, Po-Yu Wen, Tsunghsueh Wu, and Yang-Wei Lin. 2020. "Microwave-Assisted Synthesis of Chalcopyrite/Silver Phosphate Composites with Enhanced Degradation of Rhodamine B under Photo-Fenton Process" Nanomaterials 10, no. 11: 2300. https://doi.org/10.3390/nano10112300
APA StyleChang, S. -A., Wen, P. -Y., Wu, T., & Lin, Y. -W. (2020). Microwave-Assisted Synthesis of Chalcopyrite/Silver Phosphate Composites with Enhanced Degradation of Rhodamine B under Photo-Fenton Process. Nanomaterials, 10(11), 2300. https://doi.org/10.3390/nano10112300