Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of meso-5-(4-hydroxyphenyl)-10,15,20-tris(3,5-di-tert-butylphenyl)porphyrin H2L1
2.2. Synthesis of meso-[5-(4-hydroxyphenyl)-10,15,20-tris(3,5-di-tert-butylphenyl)porphyrinato] Zinc(II) ZnL1
2.3. Synthesis of Triad 1
2.4. Synthesis of Triad 2
2.5. Synthesis of 5,15-bis(3-Pyridyl)-10,20-bis(phenyl)Porphyrin H2L2
2.6. Synthesis of trans-dihydroxo-[5,15-bis(3-pyridyl)-10,20-bis(phenyl)porphyrinato]tin(IV) SnL2
2.7. Synthesis of Triad 3
2.8. Synthesis of Triad 4
3. Results and Discussion
3.1. Syntheses
3.2. Spectroscopic Characterization
3.3. Supramolecular Self-Assembly to Nanostructures
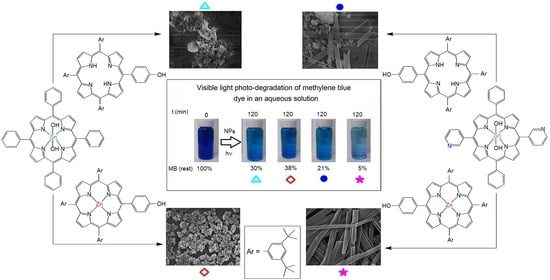
3.4. Photocatalysis for the Degradation of Methylene Blue (MB) Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.-P.; Lin, W.; Wang, X.; Cen, T.-Y.; Xie, H.; Huang, J.; Zhu, B.-Y.; Zhang, Z.; Song, A.; Hao, J.; et al. Controllable hierarchical self-assembly of porphyrin-derived supra-amphiphiles. Nat. Commun. 2019, 10, 1399–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Wang, L.; Wang, H.; Cao, R.; Wang, J.; Bai, F.; Fan, H. Self-assembled one-dimensional porphyrin nanostructures with enhanced photocatalytic hydrogen generation. Nano Lett. 2018, 18, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef] [Green Version]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 335, 737–740. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. recent advances in porphyrin-based inorganic nanoparticles for cancer treatment. Int. J. Mol. Sci. 2020, 21, 3358. [Google Scholar] [CrossRef]
- Qi, Z.-L.; Cheng, Y.-H.; Xu, Z.; Chen, M.-L. Recent advances in porphyrin-based materials for metal ions detection. Int. J. Mol. Sci. 2020, 21, 5839. [Google Scholar] [CrossRef]
- Drain, C.M.; Smeureanu, G.; Patel, S.; Gong, X.; Garno, J.; Arijeloye, J. Porphyrin nanoparticles as supramolecular systems. New J. Chem. 2006, 30, 1834–1843. [Google Scholar] [CrossRef]
- Elemans, J.A.; van Hameren, R.; Nolte, R.J.; Rowan, A.E. Molecular materials by self-assembly of porphyrins, phthalocyanines, and perylenes. Adv. Mater. 2006, 18, 1251–1266. [Google Scholar] [CrossRef]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef] [Green Version]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Li, Y.; Li, Y. Aggregate nanostructures of organic molecular materials. Acc. Chem. Res. 2010, 43, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-based nanostructures for photocatalytic applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The assembly of porphyrin systems in well-defined nanostructures: An update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef] [Green Version]
- Habets, T.; Lensen, D.; Speller, S.; Elemans, J.A. Self-assembly of covalently linked porphyrin dimers at the solid–liquid interface. Molecules 2019, 24, 3018. [Google Scholar] [CrossRef] [Green Version]
- Burrell, A.K.; Wasielewski, M.R. Porphyrin-based nanostructures: Routes to molecular electronics. J. Porphyr. Phthalocyanines 2000, 4, 401–406. [Google Scholar] [CrossRef]
- Gong, X.; Milic, T.; Xu, C.; Batteas, J.D.; Drain, C.M. Preparation and characterization of porphyrin nanoparticles. J. Am. Chem. Soc. 2002, 124, 14290–14291. [Google Scholar] [CrossRef]
- Tian, Y.; Beavers, C.M.; Busani, T.; Martin, K.E.; Jacobsen, J.L.; Mercado, B.Q.; Swartzentruber, B.S.; Swol, F.van; Medfortheg, C.J.; Shelnutt, J.A. Binary ionic porphyrin nanosheets: Electronic and light-harvesting properties regulated by crystal structure. Nanoscale 2012, 4, 1695–1700. [Google Scholar] [CrossRef]
- Hasobe, T. Photo- and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Trapani, M.; Mazzaglia, A.; Piperno, A.; Cordaro, A.; Zagami, R.; Castriciano, M.A.; Romeo, A.; Monsù Scolaro, L. Novel nanohybrids based on supramolecular assemblies of meso-tetrakis-(4-sulfonatophenyl) porphyrin J-aggregates and amine-functionalized carbon nanotubes. Nanomaterials 2020, 10, 669. [Google Scholar] [CrossRef] [Green Version]
- Lehn, J.-M. Perspectives in supramolecular chemistry—From molecular recognition towards molecular information processing and self-organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathius, J.P. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T.; Nguyen, S.T. Amphiphilic porphyrin nanocrystals: Morphology tuning and hierarchical assembly. Adv. Mater. 2008, 20, 3543–3549. [Google Scholar] [CrossRef]
- Hasobe, T.; Oki, H.; Sandanayakaa, A.S.D.; Murata, H. Sonication-assisted supramolecular nanorods of meso-diaryl-substituted porphyrins. Chem. Commun. 2008, 11, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin supramolecular 1D structures via surfactant-assisted self-assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanotubes by ionic self-assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 47, 7261–7277. [Google Scholar] [CrossRef]
- Stefanelli, M.; Mandoj, F.; Magna, G.; Lettieri, R.; Venanzi, M.; Paolesse, R.; Monti, D. The self-aggregation of porphyrins with multiple chiral centers in organic/aqueous media: The case of sugar- and steroid-porphyrin conjugates. Molecules 2020, 25, 4544. [Google Scholar] [CrossRef]
- Lu, J.; Li, Z.; An, W.; Liu, L.; Cui, W. Tuning the supramolecular structures of metal-free porphyrin via surfactant assisted self-assembly to enhance photocatalytic performance. Nanomaterials 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Spitaleri, L.; Gangemi, C.M.A.; Purrello, R.; Nicotra, G.; Trusso Sfrazzetto, G.; Casella, G.; Casarin, M.; Gulino, A. Covalently conjugated gold–porphyrin nanostructures. Nanomaterials 2020, 10, 1644. [Google Scholar] [CrossRef]
- Tian, Y.; Busani, T.; Uyeda, G.H.; Martin, K.E.; Swol, F.van; Medforth, C.J.; Montan, G.A.; Shelnutt, J.A. Hierarchical cooperative binary ionic porphyrin nanocomposites. Chem. Commun. 2012, 48, 4863–4865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Medforth, C.J.; Shelnutt, J.A. Self-assembly and self-metallization of porphyrin nanosheets. J. Am. Chem. Soc. 2007, 129, 2440–2441. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Self-metallization of photocatalytic porphyrin nanotubes. J. Am. Chem. Soc. 2004, 126, 16720–16721. [Google Scholar] [CrossRef]
- Song, Y.; Yang, Y.; Medforth, C.J.; Pereira, E.; Singh, A.K.; Xu, H.; Jiang, Y.; Brinker, C.J.; van Swol, F.; Shelnutt, J.A. Controlled synthesis of 2-D and 3-D dendritic platinum nanostructures. J. Am. Chem. Soc. 2004, 126, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, K.-M.; Ahn, T.K.; Kim, S.K.; Kim, K.S.; Kim, D.; Kim, H.-J. Novel fullerene–porphyrin–fullerene triad linked by metal axial coordination: Synthesis, X-ray crystal structure, and spectroscopic characterizations of trans-bis([60]fullerenoacetato)tin(iv) porphyrin. Chem. Commun. 2004, 22, 2594–2595. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jo, H.J.; Kim, J.; Kim, S.-Y.; Kim, D.; Kim, K. Supramolecular self-assembly of tin(IV) porphyrin channels stabilizing single-file chains of water molecules. CrystEngComm 2005, 7, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Metselaar, G.A.; Ballester, P.; de Mendoza, J. Cyclic oligomers based on complementary Zn(II) and Sn(IV)-porphyrins. New J. Chem. 2009, 33, 777–783. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. Supramolecular tetrads containing Sn(IV) porphyrin, Ru(II) porphyrin, and expanded porphyrins assembled using complementary metal−ligand interactions. Inorg. Chem. 2011, 50, 1713–1722. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Fluorescent chemosensing for aromatic compounds by supramolecular complex composed of tin(IV) porphyrin, viologen, and cucurbit[8]uril. Chem. Commun. 2019, 55, 10575–10578. [Google Scholar] [CrossRef]
- Kim, M.K.; Shee, N.K.; Lee, J.; Yoon, M.; Kim, H.-J. Photoinduced electron transfer upon supramolecular complexation of (porphyrinato) Sn-Viologen with cucurbit [7] uril. Photochem. Photobiol. Sci. 2019, 18, 1996–2002. [Google Scholar] [CrossRef]
- Kim, H.J.; Shee, N.K.; Park, K.M.; Kim, H.-J. Assembly and X-ray crystal structures of heterometallic multiporphyrins with complementary coordination between ruthenium (II) and tin (IV) porphyrins. Inorg. Chim. Acta 2019, 488, 1–7. [Google Scholar] [CrossRef]
- Jang, J.H.; Jeon, K.-S.; Oh, S.; Kim, H.-J.; Asahi, T.; Masuhara, H.; Yoon, M. Synthesis of Sn-porphyrin-intercalated trititanate nanofibers: Optoelectronic properties and photocatalytic activities. Chem. Mater. 2007, 19, 1984–1991. [Google Scholar] [CrossRef]
- Martin, K.E.; Tian, Y.; Busani, T.; Medforth, C.J.; Franco, R.; van Swol, F.; Shelnutt, J.A. Charge effects on the structure and composition of porphyrin binary ionic solids: ZnTPPS/SnTMePyP nanomaterials. Chem. Mater. 2013, 25, 441–447. [Google Scholar] [CrossRef]
- Li, C.; Park, K.-M.; Kim, H.-J. Ionic assembled hybrid nanoparticle consisting of tin(IV) porphyrin cations and polyoxomolybdate anions, and photocatalytic hydrogen production by its visible light sensitization. Inorg. Chem. Commun. 2015, 60, 8–11. [Google Scholar] [CrossRef]
- Boccalon, M.; Iengo, E.; Tecilla, P. Metal−organic transmembrane nanopores. J. Am. Chem. Soc. 2012, 134, 20310–20313. [Google Scholar] [CrossRef]
- Giribabu, L.; Rao, T.A.; Maiya, B.G. “Axial-Bonding”-type hybrid porphyrin arrays: Synthesis, spectroscopy, electrochemistry, and singlet state properties. Inorg. Chem. 1999, 38, 4971–4980. [Google Scholar] [CrossRef]
- Kumar, A.A.; Giribabu, L.; Reddy, D.R.; Maiya, B.G. New molecular arrays based on a Tin(IV) porphyrin scaffold. Inorg. Chem. 2001, 40, 6757–6766. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. Sn(IV) Porphyrin based axial-bonding type porphyrin triads containing heteroporphyrins as axial ligands. Inorg. Chem. 2010, 49, 2692–2700. [Google Scholar] [CrossRef]
- Honda, T.; Nakanishi, T.; Ohkubo, K.; Kojima, T.; Fukuzumi, S. Formation of a long-lived photoinduced electron-transfer state in an electron acceptor−donor−acceptor porphyrin triad connected by coordination bonds. J. Phys. Chem. C 2010, 114, 14290–14299. [Google Scholar] [CrossRef]
- Amati, A.; Cavigli, P.; Demitri, N.; Natali, M.; Indelli, M.T.; Iengo, E. Sn(IV) multiporphyrin arrays as tunable photoactive systems. Inorg. Chem. 2019, 58, 4399–4411. [Google Scholar] [CrossRef]
- La, D.D.; Hangarge, R.V.; Bhosale, S.V.; Ninh, H.D.; Jones, L.A.; Bhosale, S.V. Arginine-mediated self-assembly of porphyrin on graphene: A photocatalyst for degradation of dyes. Appl. Sci. 2017, 7, 643. [Google Scholar] [CrossRef] [Green Version]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid materials based on silica matrices impregnated with Pt-Porphyrin or PtNPs destined for CO2 gas detection or for wastewaters color removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezapor, E.; Eslami, A. Sensitization of magnetic TiO2 with copper(II) tetrahydroxylphenyl porphyrin for photodegradation of methylene blue by visible LED light. J. Mater. Sci. Mater. Electron. 2019, 30, 4705–4715. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Lu, Z.-Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater. 2020, 292, 109774. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Hubenko, K.; Yefimova, S.; Tkacheva, T.; Maksimchuk, P.; Borovoy, I.; Klochkov, V.; Kavok, N.; Opolonin, O.; Malyukin, Y. Reactive oxygen species generation in aqueous solutions containing GdVO4:Eu3+ nanoparticles and their complexes with methylene blue. Nanoscale Res. Lett. 2018, 13, 100. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials 2020, 10, 2314. https://doi.org/10.3390/nano10112314
Shee NK, Kim MK, Kim H-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials. 2020; 10(11):2314. https://doi.org/10.3390/nano10112314
Chicago/Turabian StyleShee, Nirmal Kumar, Min Kyoung Kim, and Hee-Joon Kim. 2020. "Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye" Nanomaterials 10, no. 11: 2314. https://doi.org/10.3390/nano10112314
APA StyleShee, N. K., Kim, M. K., & Kim, H. -J. (2020). Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials, 10(11), 2314. https://doi.org/10.3390/nano10112314