An Updated Review on Silver Nanoparticles in Biomedicine
Abstract
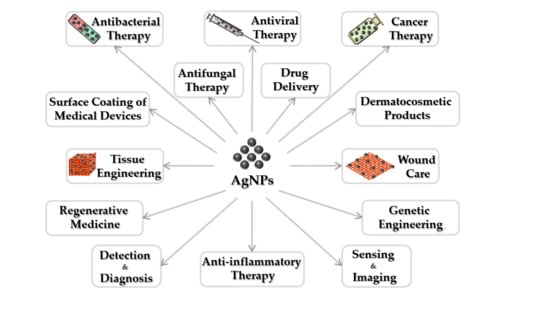
:1. Introduction
2. Toxicity of Silver Nanoparticles
3. Silver Nanoparticles for Antibacterial Applications
4. Silver Nanoparticles for Antiviral Applications
5. Silver Nanoparticles for Cancer Therapy
6. Silver Nanoparticles for Tissue Engineering
7. Silver Nanoparticles for Wound Care
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Feldman, D. Polymer nanocomposites for tissue engineering, antimicrobials and drug delivery. Biointerface Res. Appl. Chem. 2018, 8, 3153–3160. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; Kim, Y.G.; Kim, D.; Hyeon, T. Inorganic nanoparticles with enzyme-mimetic activities for biomedical applications. Coord. Chem. Rev. 2020, 403, 213092. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Keshvadi, M.; Karimi, F.; Valizadeh, S.; Valizadeh, A. Comparative study of antibacterial inhibitory effect of silver nanoparticles and garlic oil nanoemulsion with their combination. Biointerface Res. Appl. Chem. 2019, 9. [Google Scholar] [CrossRef]
- Gaafar, M.; El-Zawawy, L.; El-Temsahy, M.; Shalaby, T.I.; Hassan, A. Silver nanoparticles as a therapeutic agent in experimental cyclosporiasis. Exp. Parasitol. 2019, 207, 107772. [Google Scholar]
- Iqbal, S.; Fakhar-e-Alam, M.; Akbar, F.; Shafiq, M.; Atif, M.; Amin, N.; Ismail, M.; Hanif, A.; Farooq, W.A. Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 2019, 1189, 203–209. [Google Scholar]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Amarnath Praphakar, R.; Jeyaraj, M.; Ahmed, M.; Suresh Kumar, S.; Rajan, M. Silver nanoparticle functionalized CS-g-(CA-MA-PZA) carrier for sustainable anti-tuberculosis drug delivery. Int. J. Biol. Macromol. 2018, 118, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Yao, Q.; Yu, F.; Chen, L.; Zhang, S.; Sun, H.; Lin, J.; Fu, Y. Surface modified electrospun poly(lactic acid) fibrous scaffold with cellulose nanofibrils and Ag nanoparticles for ocular cell proliferation and antimicrobial application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110767. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shi, L.; Liu, F.; Zhang, Y.; Pang, Y.; Shen, X. Self-assembled thixotropic silver cluster hydrogel for anticancer drug release. Chem. Eng. J. 2019, 362, 650–657. [Google Scholar] [CrossRef]
- Kraeling, M.E.K.; Topping, V.D.; Keltner, Z.M.; Belgrave, K.R.; Bailey, K.D.; Gao, X.; Yourick, J.J. In vitro percutaneous penetration of silver nanoparticles in pig and human skin. Regul. Toxicol. Pharmacol. 2018, 95, 314–322. [Google Scholar] [CrossRef]
- Odeniyi, M.A.; Okumah, V.C.; Adebayo-Tayo, B.C.; Odeniyi, O.A. Green synthesis and cream formulations of silver nanoparticles of Nauclea latifolia (African peach) fruit extracts and evaluation of antimicrobial and antioxidant activities. Sustain. Chem. Pharm. 2020, 15, 100197. [Google Scholar] [CrossRef]
- Mandakhalikar, K.D.; Wang, R.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Tambyah, P.A. Restriction of in vivo infection by antifouling coating on urinary catheter with controllable and sustained silver release: A proof of concept study. BMC Infect. Dis. 2018, 18, 370. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive Surfaces of Polylactide and Silver Nanoparticles for the Prevention of Microbial Contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef] [Green Version]
- Fadlilah, D.R.; Endarko, E.; Ratnasari, A.; Hozairi, H.; Yusop, Z.; Syafiuddin, A. Enhancement of antibacterial properties of various polymers functionalized with silver nanoparticles. Biointerface Res. Appl. Chem. 2019, 10, 5592–5598. [Google Scholar] [CrossRef]
- Erring, M.; Gaba, S.; Mohsina, S.; Tripathy, S.; Sharma, R.K. Comparison of efficacy of silver-nanoparticle gel, nano-silver-foam and collagen dressings in treatment of partial thickness burn wounds. Burns 2019, 45, 1888–1894. [Google Scholar]
- Zhou, Y.; Tang, R.C. Facile and eco-friendly fabrication of AgNPs coated silk for antibacterial and antioxidant textiles using honeysuckle extract. J. Photochem. Photobiol. B Biol. 2018, 178, 463–471. [Google Scholar] [CrossRef]
- Fajar, M.N.; Endarko, E.; Rubiyanto, A.; Malek, N.A.N.N.; Hadibarata, T.; Syafiuddin, A. A green deposition method of silver nanoparticles on textiles and their antifungal activity. Biointerface Res. Appl. Chem. 2019, 10, 4902–4907. [Google Scholar] [CrossRef]
- Narasaiah, B.P.; Mandal, B.K.; Chakravarthula, S.N. Mitigation of textile industries generated pollution by agro-waste cotton peels mediated synthesized silver nanoparticles. Biointerface Res. Appl. Chem. 2018, 8, 3602–3610. [Google Scholar]
- Amini, E.; Azadfallah, M. In situ synthesis of silver nanoparticles on fiber matrix for preparing antibacterial paper. Biointerface Res. Appl. Chem. 2018, 8, 3449–3456. [Google Scholar]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag. Shelf Life 2020, 26, 100575. [Google Scholar] [CrossRef]
- Mishra, M.P.; Padhy, R.N. Antibacterial activity of green silver nanoparticles synthesized from Anogeissus acuminata against multidrug resistant urinary tract infecting bacteria in vitro and host-toxicity testing. J. Appl. Biomed. 2018, 16, 120–125. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef] [Green Version]
- Samoilova, N.A.; Krayukhina, M.A.; Popov, D.A.; Anuchina, N.M.; Piskarev, V.E. 3′-sialyllactose-decorated silver nanoparticles: Lectin binding and bactericidal properties. Biointerface Res. Appl. Chem. 2018, 8, 3095–3099. [Google Scholar]
- Lampe, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombradi, Z.; Hajdu, P.; Hegedus, V.; Racz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef] [Green Version]
- Rafique, M.; Rafique, M.S.; Kalsoom, U.; Afzal, A.; Butt, S.H.; Usman, A. Laser ablation synthesis of silver nanoparticles in water and dependence on laser nature. Opt. Quantum Electron. 2019, 51. [Google Scholar] [CrossRef]
- Han, H.J.; Yu, T.; Kim, W.S.; Im, S.H. Highly reproducible polyol synthesis for silver nanocubes. J. Cryst. Growth 2017, 469, 48–53. [Google Scholar] [CrossRef]
- Kuntyi, О.І.; Kytsya, А.R.; Mertsalo, I.P.; Mazur, А.S.; Zozula, G.І.; Bazylyak, L.I.; Тopchak, R.V. Electrochemical synthesis of silver nanoparticles by reversible current in solutions of sodium polyacrylate. Colloid Polym. Sci. 2019, 297, 689–695. [Google Scholar] [CrossRef]
- Ali, G.W.; Abd El-Moez, S.H.; Abdel-Fattah, W.A. Synthesis and characterization of nontoxic silver nano-particles with preferential bactericidal activity. Biointerface Res. Appl. Chem. 2019, 9, 4617–4623. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Salmiati, S.; Marpongahtun, M.; Salim, M.R.; Lolo, J.A.; Syafiuddin, A. Green Synthesis of Silver Nanoparticles Using Muntingia calabura Leaf Extract and Evaluation of Antibacterial Activities. Biointerface Res. Appl. Chem. 2020, 10, 6253–6261. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kumar, V.; Gundampati, R.K.; Malviya, M.; Hasan, S.H.; Jagannadham, M.V. Biosynthesis of silver nanoparticles from the novel strain of Streptomyces Sp. BHUMBU-80 with highly efficient electroanalytical detection of hydrogen peroxide and antibacterial activity. J. Environ. Chem. Eng. 2017, 5, 5624–5635. [Google Scholar] [CrossRef]
- Shahzad, A.; Saeed, H.; Iqtedar, M.; Hussain, S.Z.; Kaleem, A.; Abdullah, R.; Sharif, S.; Naz, S.; Saleem, F.; Aihetasham, A.; et al. Size-Controlled Production of Silver Nanoparticles by Aspergillus fumigatus BTCB10: Likely Antibacterial and Cytotoxic Effects. J. Nanomater. 2019, 2019, 5168698. [Google Scholar] [CrossRef] [Green Version]
- Mohler, J.S.; Sim, W.; Blaskovich, M.A.T.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.; Patil, S.; Mullani, S.; Delekar, S. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar]
- Sharma, N.; Phutela, K.; Goel, A.; Soni, S.; Batra, N. Exploring the bacterial based silver nanoparticle for their possible application as disinfectants. Biointerface Res. Appl. Chem. 2018, 8, 3100–3104. [Google Scholar]
- Montano, E.; Vivo, M.; Guarino, A.M.; di Martino, O.; Di Luccia, B.; Calabro, V.; Caserta, S.; Pollice, A. Colloidal Silver Induces Cytoskeleton Reorganization and E-Cadherin Recruitment at Cell-Cell Contacts in HaCaT Cells. Pharmaceuticals 2019, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.J.; Menking-Colby, M.N.; Humphrey, K.A.; Victory, J.H.; Kipps, D.W.; Spitzer, N. Involvement of β-catenin in cytoskeleton disruption following adult neural stem cell exposure to low-level silver nanoparticles. Neurotoxicology 2019, 71, 102–112. [Google Scholar]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Kim, J.H.; Hong, K. Cytotoxic Potential and Molecular Pathway Analysis of Silver Nanoparticles in Human Colon Cancer Cells HCT116. Int. J. Mol. Sci. 2018, 19, 2269. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Mendhulkar, V.D. Antiproliferative activity of Camellia sinensis mediated silver nanoparticles on three different human cancer cell lines. J. Cancer Res. Ther. 2018, 14, 1316. [Google Scholar] [PubMed]
- Mei, L.; Xu, Z.; Shi, Y.; Lin, C.; Jiao, S.; Zhang, L.; Li, P. Multivalent and synergistic chitosan oligosaccharide-Ag nanocomposites for therapy of bacterial infection. Sci. Rep. 2020, 10, 10011. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Duran, N.; Diez, M.C.; Martinez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Selvakumar, P.; Sithara, R.; Viveka, K.; Sivashanmugam, P. Green synthesis of silver nanoparticles using leaf extract of Acalypha hispida and its application in blood compatibility. J. Photochem. Photobiol. B Biol. 2018, 182, 52–61. [Google Scholar] [CrossRef]
- Jayeoye, T.J.; Nwabor, O.F.; Rujiralai, T. Synthesis of highly stable and dispersed silver nanoparticles/poly(vinyl alcohol-co-ethylene glycol)/poly(3-aminophenyl boronic acid) nanocomposite: Characterization and antibacterial, hemolytic and cytotoxicity studies. J. Ind. Eng. Chem. 2020, 89, 288–300. [Google Scholar] [CrossRef]
- Asghar, M.A.; Yousuf, R.I.; Shoaib, M.H.; Asghar, M.A. Antibacterial, anticoagulant and cytotoxic evaluation of biocompatible nanocomposite of chitosan loaded green synthesized bioinspired silver nanoparticles. Int. J. Biol. Macromol. 2020, 160, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, D.H.; Lim, H.J.; Kwon, T.; Choi, J.S.; Jeong, S.; Choi, I.H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef]
- Dalzon, B.; Torres, A.; Diemer, H.; Ravanel, S.; Collin-Faure, V.; Pernet-Gallay, K.; Jouneau, P.H.; Bourguignon, J.; Cianférani, S.; Carrière, M.; et al. How reversible are the effects of silver nanoparticles on macrophages? A proteomic-instructed view. Environ. Sci. Nano 2019, 6, 3133–3157. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.; Chhibber, S. Biofunctionalization of Silver Nanoparticles With Lactonase Leads to Altered Antimicrobial and Cytotoxic Properties. Front. Mol. Biosci. 2019, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Morales, J.D.M.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [PubMed]
- Pavicic, I.; Milic, M.; Pongrac, I.M.; Brkic Ahmed, L.; Matijevic Glavan, T.; Ilic, K.; Zapletal, E.; Curlin, M.; Mitrecic, D.; Vinkovic Vrcek, I. Neurotoxicity of silver nanoparticles stabilized with different coating agents: In vitro response of neuronal precursor cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 136, 110935. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, N.; Liu, Q.S.; Zhang, J.; Zhou, Q.; Jiang, G. Silver nanoparticles induce size-dependent and particle-specific neurotoxicity to primary cultures of rat cerebral cortical neurons. Ecotoxicol. Environ. Saf. 2020, 198, 110674. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bahuguna, A.; Krishnan, M.; Shukla, S.; Lee, H.; Min, S.H.; Choi, D.K.; Cho, Y.; Bajpai, V.K.; Huh, Y.S.; et al. The effect of biogenic manufactured silver nanoparticles on human endothelial cells and zebrafish model. Sci. Total Environ. 2019, 679, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar]
- Pittol, M.; Tomacheski, D.; Simões, D.N.; Ribeiro, V.F.; Santana, R.M.C. Evaluation of the Toxicity of Silver/Silica and Titanium Dioxide Particles in Mammalian Cells. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Verkhovskii, R.; Kozlova, A.; Atkin, V.; Kamyshinsky, R.; Shulgina, T.; Nechaeva, O. Physical properties and cytotoxicity of silver nanoparticles under different polymeric stabilizers. Heliyon 2019, 5, e01305. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, P.; Ravichandran, A.; Manoharan, V.; Muthukaruppan, R.; Somasundaram, S.; Pandi, B.; Krishnan, A.; Marimuthu, P.N.; Somasundaram, S.S.N.; You, S. Synthesis of Oldenlandia umbellata stabilized silver nanoparticles and their antioxidant effect, antibacterial activity, and bio-compatibility using human lung fibroblast cell line WI-38. Process Biochem. 2019, 86, 196–204. [Google Scholar] [CrossRef]
- Lyu, Y.; Yu, M.; Liu, Q.; Zhang, Q.; Liu, Z.; Tian, Y.; Li, D.; Changdao, M. Synthesis of silver nanoparticles using oxidized amylose and combination with curcumin for enhanced antibacterial activity. Carbohydr. Polym. 2020, 230, 115573. [Google Scholar]
- Ge, M.; Li, J.; Song, S.; Meng, N.; Zhou, N. Development and antibacterial performance of silver nanoparticles-lecithin modified montmorillonite nanoparticle hybrid. Appl. Clay Sci. 2019, 183, 105334. [Google Scholar] [CrossRef]
- Roy, A.; Joshi, M.; Butola, B.S.; Ghosh, S. Evaluation of biological and cytocompatible properties in nano silver-clay based polyethylene nanocomposites. J. Hazard. Mater. 2020, 384, 121309. [Google Scholar] [CrossRef] [PubMed]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Nordmeyer, D.; Sengstock, C.; Ahlberg, S.; Diendorf, J.; Raabe, J.; Epple, M.; Köller, M.; Lademann, J.; Vogt, A.; et al. Shape-Dependent Dissolution and Cellular Uptake of Silver Nanoparticles. Langmuir 2018, 34, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; Germano-Costa, T.; Pasquoto-Stigliani, T.; Fraceto, L.F.; Lima, R.d. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Khalil, N.M.; Abd El-Ghany, M.N.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef]
- Huy, T.Q.; Thanh, N.T.H.; Thuy, N.T.; Van Chung, P.; Hung, P.N.; Le, A.T.; Hanh, N.T.H. Cytotoxicity and antiviral activity of electrochemical–synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57. [Google Scholar]
- Ferdous, Z.; Al-Salam, S.; Greish, Y.E.; Ali, B.H.; Nemmar, A. Pulmonary exposure to silver nanoparticles impairs cardiovascular homeostasis: Effects of coating, dose and time. Toxicol. Appl. Pharmacol. 2019, 367, 36–50. [Google Scholar] [CrossRef]
- Ontong, J.C.; Paosen, S.; Shankar, S.; Voravuthikunchai, S.P. Eco-friendly synthesis of silver nanoparticles using Senna alata bark extract and its antimicrobial mechanism through enhancement of bacterial membrane degradation. J. Microbiol. Methods 2019, 165, 105692. [Google Scholar]
- Hussein, J.; El-Naggar, M.E.; Fouda, M.M.G.; Morsy, O.M.; Ajarem, J.S.; Almalki, A.M.; Allam, A.A.; Mekawi, E.M. The efficiency of blackberry loaded AgNPs, AuNPs and Ag@AuNPs mediated pectin in the treatment of cisplatin-induced cardiotoxicity in experimental rats. Int. J. Biol. Macromol. 2020, 159, 1084–1093. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzău, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells In Vitro. J. Nanomater. 2019, 2019, 6090259. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Harper, B.J.; Harper, S.L. Differential dissolution and toxicity of surface functionalized silver nanoparticles in small-scale microcosms: Impacts of community complexity. Environ. Sci. Nano 2017, 4, 359–372. [Google Scholar]
- Heshmati, M.; Arbabi Bidgoli, S.; Khoei, S.; Mahmoudzadeh, A.; Sorkhabadi, S.M.R. Cytotoxicity and genotoxicity of silver nanoparticles in Chinese Hamster ovary cell line (CHO-K1) cells. Nucleus 2019, 62, 221–225. [Google Scholar] [CrossRef]
- Tang, J.; Chen, B.; Cai, E.; Liu, W.; Jiang, J.; Chen, F.; Shan, X.; Zhang, H. Mechanisms of silver nanoparticles-induced cytotoxicity and apoptosis in rat tracheal epithelial cells. J. Toxicol. Sci. 2019, 44, 155–165. [Google Scholar]
- Xue, Y.; Wang, J.; Huang, Y.; Gao, X.; Kong, L.; Zhang, T.; Tang, M. Comparative cytotoxicity and apoptotic pathways induced by nanosilver in human liver HepG2 and L02 cells. Hum. Exp. Toxicol. 2018, 37, 1293–1309. [Google Scholar] [CrossRef]
- Plackal Adimuriyil George, B.; Kumar, N.; Abrahamse, H.; Ray, S.S. Apoptotic efficacy of multifaceted biosynthesized silver nanoparticles on human adenocarcinoma cells. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Yin, N.; Yang, R.; Liang, S.; Liang, S.; Faiola, F. Silver nanoparticles (AgNPs) and AgNO3 perturb the specification of human hepatocyte-like cells and cardiomyocytes. Sci. Total Environ. 2020, 725, 138433. [Google Scholar] [CrossRef]
- Jarrar, Y.; Al-Doaiss, A.; Alfaifi, M.; Shati, A.; Al-Kahtani, M.; Jarrar, B. The influence of five metallic nanoparticles on the expression of major drug-metabolizing enzyme genes with correlation of inflammation in mouse livers. Environ. Toxicol. Pharmacol. 2020, 80, 103449. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Bolfa, P.; Clichici, S.; Filip, G.A. Modulatory effects of Cornus sanguinea L. mediated green synthesized silver nanoparticles on oxidative stress, COX-2/NOS2 and NFkB/pNFkB expressions in experimental inflammation in Wistar rats. Mater. Sci. Eng. C 2020, 110, 110709. [Google Scholar] [CrossRef]
- Elsharawy, K.; Abou-Dobara, M.; El-Gammal, H.; Hyder, A. Chitosan coating does not prevent the effect of the transfer of green silver nanoparticles biosynthesized by Streptomyces malachitus into fetuses via the placenta. Reprod. Biol. 2020, 20, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Turcotte, P.; Gagnon, C.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. The influence of surface coatings on the toxicity of silver nanoparticle in rainbow trout. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 226, 108623. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Zucker, R.M.; Ortenzio, J.; Degn, L.L.; Lerner, J.M.; Boyes, W.K. Biophysical comparison of four silver nanoparticles coatings using microscopy, hyperspectral imaging and flow cytometry. PLoS ONE 2019, 14, e0219078. [Google Scholar] [CrossRef] [Green Version]
- Docea, A.O.; Calina, D.; Buga, A.M.; Zlatian, O.; Paoliello, M.M.B.; Mogosanu, G.D.; Streba, C.T.; Popescu, E.L.; Stoica, A.E.; Bîrcă, A.C.; et al. The Effect of Silver Nanoparticles on Antioxidant/Pro-Oxidant Balance in a Murine Model. Int. J. Mol. Sci. 2020, 21, 1233. [Google Scholar] [CrossRef] [Green Version]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Silva, E.A.; de Camargo, E.R.; Gomes-Filho, J.E.; de Oliveira, S.H.P.; Barbosa, D.B. Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm. Mater. Sci. Eng. C 2021, 118, 111341. [Google Scholar] [CrossRef]
- Kern, W.V.; Rieg, S. Burden of bacterial bloodstream infection—A brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef]
- Saldana, C.S.; Vyas, D.A.; Wurcel, A.G. Soft Tissue, Bone, and Joint Infections in People Who Inject Drugs. Infect. Dis. Clin. N. Am. 2020, 34, 495–509. [Google Scholar] [CrossRef]
- Koulenti, D.; Song, A.; Ellingboe, A.; Abdul-Aziz, M.H.; Harris, P.; Gavey, E.; Lipman, J. Infections by multidrug-resistant Gram-negative Bacteria: What’s new in our arsenal and what’s in the pipeline? Int. J. Antimicrob. Agents 2019, 53, 211–224. [Google Scholar] [CrossRef]
- Escolà-Vergé, L.; Los-Arcos, I.; Almirante, B. New antibiotics for the treatment of infections by multidrug-resistant microorganisms. Med. Clínica 2020, 154, 351–357. [Google Scholar] [CrossRef]
- Shahriari, S.; Monajjemi, M.; Zare, K. Penetrating to cell membrane bacteria by the efficiency of various antibiotics (clindamycin, metronidazole, azithromycin, sulfamethoxazole, baxdela, ticarcillin, and clavulanic acid) using S-NICS theory. Biointerface Res. Appl. Chem. 2018, 8, 3219–3223. [Google Scholar]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. Dose-dependent antimicrobial activity of silver nanoparticles on polycaprolactone fibers against gram-positive and gram-negative bacteria. J. Nanomater. 2017, 2017, 4752314. [Google Scholar]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [PubMed]
- Shaaban, M.; Elgaml, A.; Habib, E.E. Biotechnological applications of quorum sensing inhibition as novel therapeutic strategies for multidrug resistant pathogens. Microb. Pathog. 2019, 127, 138–143. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007(-)2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Hadrup, N.; Sharma, A.K.; Loeschner, K.; Jacobsen, N.R. Pulmonary toxicity of silver vapours, nanoparticles and fine dusts: A review. Regul. Toxicol. Pharmacol. 2020, 115, 104690. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.L.; Capjak, I.; Vrcek, I.V.; Bondarenko, O.M.; Kurvet, I.; Vija, H.; Ivask, A.; Kasemets, K.; Kahru, A. Antimicrobial potency of differently coated 10 and 50 nm silver nanoparticles against clinically relevant bacteria Escherichia coli and Staphylococcus aureus. Colloids Surf. B Biointerfaces 2018, 170, 401–410. [Google Scholar] [CrossRef]
- Huang, X.; Bao, X.; Liu, Y.; Wang, Z.; Hu, Q. Catechol-Functional Chitosan/Silver Nanoparticle Composite as a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. Sci. Rep. 2017, 7, 1860. [Google Scholar] [CrossRef]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar]
- Li, W.R.; Sun, T.L.; Zhou, S.L.; Ma, Y.K.; Shi, Q.S.; Xie, X.B.; Huang, X.M. A comparative analysis of antibacterial activity, dynamics, and effects of silver ions and silver nanoparticles against four bacterial strains. Int. Biodeterior. Biodegrad. 2017, 123, 304–310. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef] [Green Version]
- Chang, B.M.; Pan, L.; Lin, H.H.; Chang, H.C. Nanodiamond-supported silver nanoparticles as potent and safe antibacterial agents. Sci. Rep. 2019, 9, 13164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinteros, M.A.; Cano Aristizabal, V.; Dalmasso, P.R.; Paraje, M.G.; Paez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. Int. J. Publ. Assoc. Bibra 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Viviana, C.A.; Onnainty, R.; Mary, V.S.; Theumer, M.G.; Granero, G.E.; Paraje, M.G.; Paez, P.L. Biosynthesized silver nanoparticles: Decoding their mechanism of action in Staphylococcus aureus and Escherichia coli. Int. J. Biochem. Cell Biol. 2018, 104, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Rudakiya, D.M.; Pawar, K. Bactericidal potential of silver nanoparticles synthesized using cell-free extract of Comamonas acidovorans: In vitro and in silico approaches. 3 Biotech. 2017, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Tamiyakul, H.; Roytrakul, S.; Jaresitthikunchai, J.; Phaonakrop, N.; Tanasupawat, S.; Warisnoicharoen, W. Changes in protein patterns of Staphylococcus aureus and Escherichia coli by silver nanoparticles capped with poly (4-styrenesulfonic acid-co-maleic acid) polymer. Asian Biomed. 2019, 13, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Roseli Salomoni, P.L. Maria Filomena de Andrade Rodrigues. Antibacterial Activity of Silver Nanoparticles (AgNPs) in Staphylococcus aureus and Cytotoxicity in Mammalian Cells. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs, 1st ed.; Formatex Research Center: Badajos, Spain, 2015. [Google Scholar]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Effect of the size and shape of silver nanoparticles on bacterial growth and metabolism by monitoring optical density and fluorescence intensity. Biotechnol. Bioprocess. Eng. 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Qasim, M.; Udomluck, N.; Chang, J.; Park, H.; Kim, K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomed. 2018, 13, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zhou, S.; Fu, Y.; Wang, Y.; Mi, J.; Lu, T.; Wang, X.; Lu, C. Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110735. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Adv. Powder Technol. 2018, 29, 407–415. [Google Scholar] [CrossRef]
- Korshed, P.; Li, L.; Liu, Z.; Mironov, A.; Wang, T. Size-dependent antibacterial activity for laser-generated silver nanoparticles. J. Interdiscip. Nanomed. 2019, 4, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Perveen, S.; Ali, M.; Jiao, T.; Sharma, A.S.; Hassan, H.; Devaraj, S.; Li, H.; Chen, Q. Bioinspired morphology-controlled silver nanoparticles for antimicrobial application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110421. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Pandey, S.; Giri, V.P.; Bhattacharya, A.; Shukla, R.; Mishra, A.; Nautiyal, C.S. Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb. Pathog. 2017, 105, 346–355. [Google Scholar] [CrossRef]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Kora, A.J.; Sashidhar, R. Biogenic silver nanoparticles synthesized with rhamnogalacturonan gum: Antibacterial activity, cytotoxicity and its mode of action. Arab. J. Chem. 2018, 11, 313–323. [Google Scholar]
- Rahman, A.U.; Khan, A.U.; Yuan, Q.; Wei, Y.; Ahmad, A.; Ullah, S.; Khan, Z.U.H.; Shams, S.; Tariq, M.; Ahmad, W. Tuber extract of Arisaema flavum eco-benignly and effectively synthesize silver nanoparticles: Photocatalytic and antibacterial response against multidrug resistant engineered E. coli QH4. J. Photochem. Photobiol. B Biol. 2019, 193, 31–38. [Google Scholar] [CrossRef]
- Rawat, V.; Sharma, A.; Bhatt, V.P.; Pratap Singh, R.; Maurya, I.K. Sunlight mediated green synthesis of silver nanoparticles using Polygonatum graminifolium leaf extract and their antibacterial activity. Mater. Today Proc. 2020, 29, 911–916. [Google Scholar] [CrossRef]
- Van Viet, P.; Sang, T.T.; Bich, N.H.N.; Thi, C.M. An improved green synthesis method and Escherichia coli antibacterial activity of silver nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 182, 108–114. [Google Scholar] [CrossRef]
- Hileuskaya, K.; Ladutska, A.; Kulikouskaya, V.; Kraskouski, A.; Novik, G.; Kozerozhets, I.; Kozlovskiy, A.; Agabekov, V. ‘Green’ approach for obtaining stable pectin-capped silver nanoparticles: Physico-chemical characterization and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124141. [Google Scholar] [CrossRef]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C.; Tangboriboonrat, P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pan, Y.; Li, N.; Wang, Q.; Chen, Y.; Phisalaphong, M.; Chen, H. Antibacterial and cytotoxic activities of a green synthesized silver nanoparticles using corn silk aqueous extract. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124827. [Google Scholar] [CrossRef]
- Das, P.; Ghosal, K.; Jana, N.K.; Mukherjee, A.; Basak, P. Green synthesis and characterization of silver nanoparticles using belladonna mother tincture and its efficacy as a potential antibacterial and anti-inflammatory agent. Mater. Chem. Phys. 2019, 228, 310–317. [Google Scholar] [CrossRef]
- Manukumar, H.M.; Yashwanth, B.; Umesha, S.; Venkateswara Rao, J. Biocidal mechanism of green synthesized thyme loaded silver nanoparticles (GTAgNPs) against immune evading tricky methicillin-resistant Staphylococcus aureus 090 (MRSA090) at a homeostatic environment. Arab. J. Chem. 2020, 13, 1179–1197. [Google Scholar] [CrossRef]
- Alsamhary, K.I. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Zhang, H.; Ashraf, M.; Fang, H.; Zeng, X.; Wu, Y.; Khurshid, M.; Zhao, L.; He, Z. Antibacterial and antioxidant activity of exopolysaccharide mediated silver nanoparticle synthesized by Lactobacillus brevis isolated from Chinese koumiss. Colloids Surf. B Biointerfaces 2020, 186, 110734. [Google Scholar] [CrossRef]
- Sabry, N.M.; Abdel-Gawad, F.K.; Bassem, S.M.; Nassar, H.F.; El-Taweel, G.E.; Okasha, A.; Ibrahim, M. Interaction between nano silver and bacteria: Modeling approach. Biointerface Res. Appl. Chem. 2018, 8, 3570–3574. [Google Scholar]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef]
- Mahjouri, S.; Movafeghi, A.; Divband, B.; Kosari-Nasab, M.; Kazemi, E.M. Assessing the toxicity of silver nanoparticles in cell suspension culture of nicotiana tabacum. Biointerface Res. Appl. Chem. 2018, 8, 3252–3258. [Google Scholar]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Ramesh, A.V.; Devi, D.R.; Battu, G.; Basavaiah, K. A Facile plant mediated synthesis of silver nanoparticles using an aqueous leaf extract of Ficus hispida Linn. f. for catalytic, antioxidant and antibacterial applications. S. Afr. J. Chem. Eng. 2018, 26, 25–34. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Pallela, P.; Ummey, S.; Ruddaraju, L.K.; Pammi, S.V.N.; Yoon, S.G. Ultra Small, mono dispersed green synthesized silver nanoparticles using aqueous extract of Sida cordifolia plant and investigation of antibacterial activity. Microb. Pathog. 2018, 124, 63–69. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.B.; Yun, H.S.; Lee, C.G. Temperature-dependent green biosynthesis and characterization of silver nanoparticles using balloon flower plants and their antibacterial potential. J. Mol. Struct. 2019, 1177, 302–309. [Google Scholar] [CrossRef]
- Rafinska, K.; Pomastowski, P.; Buszewski, B. Study of Bacillus subtilis response to different forms of silver. Sci. Total Environ. 2019, 661, 120–129. [Google Scholar] [CrossRef]
- Ashraf, A.; Zafar, S.; Zahid, K.; Salahuddin Shah, M.; Al-Ghanim, K.A.; Al-Misned, F.; Mahboob, S. Synthesis, characterization, and antibacterial potential of silver nanoparticles synthesized from Coriandrum sativum L. J. Infect. Public Health 2019, 12, 275–281. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R. Evaluation of Antibacterial Efficacy of Fungal-Derived Silver Nanoparticles against Enterococcus faecalis. Contemp. Clin. Dent. 2018, 9, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R. Antibacterial Efficacy of Biosynthesized Silver Nanoparticles against Enterococcus faecalis Biofilm: An in vitro Study. Contemp. Clin. Dent. 2018, 9, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Hidayat, H.; Nugroho, B.H.; Husein, S. Ultrasound-assisted biosynthesis of silver and gold nanoparticles using Clitoria ternatea flower. S. Afr. J. Chem. Eng. 2020, 34, 97–106. [Google Scholar] [CrossRef]
- Ameen, F.; Srinivasan, P.; Selvankumar, T.; Kamala-Kannan, S.; Al Nadhari, S.; Almansob, A.; Dawoud, T.; Govarthanan, M. Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg. Chem. 2019, 88, 102970. [Google Scholar] [CrossRef] [PubMed]
- Baruah, D.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Alpinia nigra fruits mediated synthesis of silver nanoparticles and their antimicrobial and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2019, 201, 111649. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Redhwan, A. Cytotoxic effect of green silver nanoparticles against ampicillin-resistant Klebsiella pneumoniae. RSC Adv. 2020, 10, 21136–21146. [Google Scholar] [CrossRef]
- Dolatabadi, A.; Noorbazargan, H.; Khayam, N.; Moulavi, P.; Zamani, N.; Asghari Lalami, Z.; Ashrafi, F. Ecofriendly Biomolecule-Capped Bifidobacterium bifidum-Manufactured Silver Nanoparticles and Efflux Pump Genes Expression Alteration in Klebsiella pneumoniae. Microb. Drug Resist. 2020. [Google Scholar] [CrossRef]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M.; et al. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella pneumoniae. BioMed Res. Int. 2020, 2020, 6398165. [Google Scholar] [CrossRef] [Green Version]
- Alfuraydi, A.A.; Devanesan, S.; Al-Ansari, M.; AlSalhi, M.S.; Ranjitsingh, A.J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2019, 192, 83–89. [Google Scholar] [CrossRef]
- Küp, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater. Sci. Eng. C 2020, 107, 110207. [Google Scholar] [CrossRef]
- Kumar, S.; Shahid, M.; Khan, M.A.; Kumar, N.; Khan, H.M. Biosynthesis of silver nanoparticles from Phyllanthus niruri leaf extracts and its antibacterial activity against antibiotics-resistant clinical isolates. Pathology 2020, 52, S127. [Google Scholar] [CrossRef] [Green Version]
- Ciepluch, K.; Skrzyniarz, K.; Barrios-Gumiel, A.; Quintana, S.; Sánchez-Nieves, J.; de la Mata, F.J.; Maciejewska, B.; Drulis-Kawa, Z.; Arabski, M. Dendronized Silver Nanoparticles as Bacterial Membrane Permeabilizers and Their Interactions With P. aeruginosa Lipopolysaccharides, Lysozymes, and Phage-Derived Endolysins. Front. Microbiol. 2019, 10, 2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Rolim, W.R.; Pelegrino, M.T.; de Araújo Lima, B.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Chandhru, M.; Logesh, R.; Rani, S.K.; Ahmed, N.; Vasimalai, N. One-pot green route synthesis of silver nanoparticles from jack fruit seeds and their antibacterial activities with escherichia coli and salmonella bacteria. Biocatal. Agric. Biotechnol. 2019, 20, 101241. [Google Scholar] [CrossRef]
- Lotha, R.; Sundaramoorthy, N.S.; Shamprasad, B.R.; Nagarajan, S.; Sivasubramanian, A. Plant nutraceuticals (Quercetrin and Afzelin) capped silver nanoparticles exert potent antibiofilm effect against food borne pathogen Salmonella enterica serovar Typhi and curtail planktonic growth in zebrafish infection model. Microb. Pathog. 2018, 120, 109–118. [Google Scholar] [CrossRef]
- Neethu, S.; Midhun, S.J.; Sunil, M.A.; Soumya, S.; Radhakrishnan, E.K.; Jyothis, M. Efficient visible light induced synthesis of silver nanoparticles by Penicillium polonicum ARA 10 isolated from Chetomorpha antennina and its antibacterial efficacy against Salmonella enterica serovar Typhimurium. J. Photochem. Photobiol. B Biol. 2018, 180, 175–185. [Google Scholar] [CrossRef]
- Jemilugba, O.T.; Sakho, E.H.M.; Parani, S.; Mavumengwana, V.; Oluwafemi, O.S. Green synthesis of silver nanoparticles using Combretum erythrophyllum leaves and its antibacterial activities. Colloid Interface Sci. Commun. 2019, 31, 100191. [Google Scholar] [CrossRef]
- Hashim, N.; Paramasivam, M.; Tan, J.S.; Kernain, D.; Hussin, M.H.; Brosse, N.; Gambier, F.; Raja, P.B. Green mode synthesis of silver nanoparticles using Vitis vinifera’s tannin and screening its antimicrobial activity/apoptotic potential versus cancer cells. Mater. Today Commun. 2020, 25, 101511. [Google Scholar] [CrossRef]
- Rangayasami, A.; Kannan, K.; Joshi, S.; Subban, M. Bioengineered silver nanoparticles using Elytraria acaulis (L.f.) Lindau leaf extract and its biological applications. Biocatal. Agric. Biotechnol. 2020, 27, 101690. [Google Scholar] [CrossRef]
- Ramadan, M.A.; Shawkey, A.E.; Rabeh, M.A.; Abdellatif, A.O. Promising antimicrobial activities of oil and silver nanoparticles obtained from Melaleuca alternifolia leaves against selected skin-infecting pathogens. J. Herb. Med. 2020, 20, 100289. [Google Scholar] [CrossRef]
- Dutta, T.; Ghosh, N.N.; Das, M.; Adhikary, R.; Mandal, V.; Chattopadhyay, A.P. Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: Experimental and theoretical studies. J. Environ. Chem. Eng. 2020, 8, 104019. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Gonçalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Gurumallesh Prabu, H. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Sholkamy, E.N.; Ahamd, M.S.; Yasser, M.M.; Eslam, N. Anti-microbiological activities of bio-synthesized silver Nano-stars by Saccharopolyspora hirsuta. Saudi J. Biol. Sci. 2019, 26, 195–200. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Abulateefeh, S.R.; Alkilany, A.M. Synergistic antibacterial activity of silver nanoparticles and hydrogen peroxide. PLoS ONE 2019. [Google Scholar] [CrossRef] [Green Version]
- Perugini Biasi-Garbin, R.; Saori Otaguiri, E.; Morey, A.T.; Fernandes da Silva, M.; Belotto Morguette, A.E.; Armando Contreras Lancheros, C.; Kian, D.; Perugini, M.R.E.; Nakazato, G.; Durán, N. Effect of eugenol against Streptococcus agalactiae and synergistic interaction with biologically produced silver nanoparticles. Evid. Based Complement. Altern. Med. 2015, 2015, 861497. [Google Scholar]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater. Sci. Eng. C 2019, 99, 255–263. [Google Scholar] [CrossRef]
- Qaralleh, H.; Khleifat, K.M.; Al-Limoun, M.O.; Alzedaneen, F.Y.; Al-Tawarah, N. Antibacterial and synergistic effect of biosynthesized silver nanoparticles using the fungi Tritirachium oryzae W5H with essential oil of Centaurea damascena to enhance conventional antibiotics activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025016. [Google Scholar] [CrossRef]
- Kaur, A.; Preet, S.; Kumar, V.; Kumar, R.; Kumar, R. Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Colloids Surf. B Biointerfaces 2019, 176, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Malik, M.M.; Qureshi, M.S. Study of Synergistic Effects of Antibiotics And Triangular Shaped Silver Nanoparticles, Synthesized Using UV-Light Irradiation, on S. Aureus and P. Aeruginosa. Mater. Today Proc. 2019, 18, 920–927. [Google Scholar] [CrossRef]
- Farjadian, F.; Akbarizadeh, A.R.; Tayebi, L. Synthesis of novel reducing agent for formation of metronidazole-capped silver nanoparticle and evaluating antibacterial efficiency in gram-positive and gram-negative bacteria. Heliyon 2020, 6, e04747. [Google Scholar] [CrossRef]
- Nishanthi, R.; Malathi, S.; John Paul, S.; Palani, P. Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 693–707. [Google Scholar] [CrossRef]
- Thomas, R.; Jishma, P.; Snigdha, S.; Soumya, K.R.; Mathew, J.; Radhakrishnan, E.K. Enhanced antimicrobial efficacy of biosynthesized silver nanoparticle based antibiotic conjugates. Inorg. Chem. Commun. 2020, 117, 107978. [Google Scholar] [CrossRef]
- Murei, A.; Ayinde, W.B.; Gitari, M.W.; Samie, A. Functionalization and antimicrobial evaluation of ampicillin, penicillin and vancomycin with Pyrenacantha grandiflora Baill and silver nanoparticles. Sci. Rep. 2020, 10, 11596. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.A.M.; Mohammad, A.A.-H.; Harraz, F.A.; Ahsan, M.F. Biologically Synthesized Silver Nanoparticles for Enhancing Tetracycline Activity Against Staphylococcus aureus and Klebsiella pneumoniae. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Gurunathan, S. Rapid biological synthesis of silver nanoparticles and their enhanced antibacterial effects against Escherichia fergusonii and Streptococcus mutans. Arab. J. Chem. 2019, 12, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Al-Sharqi, A.; Apun, K.; Vincent, M.; Kanakaraju, D.; Bilung, L.M. Enhancement of the Antibacterial Efficiency of Silver Nanoparticles against Gram-Positive and Gram-Negative Bacteria Using Blue Laser Light. Int. J. Photoenergy 2019, 2019, 2528490. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.T.P.; Petri, M.V.; Valencia, E.Y.; Camargo, P.H.C.; de Torresi, S.I.C.; Spira, B. Visible light plasmon excitation of silver nanoparticles against antibiotic-resistant Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2020, 31, 101908. [Google Scholar] [CrossRef]
- Szymanski, C.M.; Schnaar, R.L.; Aebi, M. Bacterial and viral infections. In Essentials of Glycobiology [Internet], 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Pezhouh, M.K.; Yang, G.Y. Viral infections of the gastrointestinal tract. Diagn. Histopathol. 2018, 24, 487–492. [Google Scholar] [CrossRef]
- Baz, M.; Boivin, G. Antiviral Agents in Development for Zika Virus Infections. Pharmaceuticals 2019, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Sreekanth, T.; Nagajyothi, P.; Muthuraman, P.; Enkhtaivan, G.; Vattikuti, S.; Tettey, C.; Kim, D.H.; Shim, J.; Yoo, K. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018, 188, 6–11. [Google Scholar]
- Mori, Y.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar]
- Alghrair, Z.K.; Fernig, D.G.; Ebrahimi, B. Enhanced inhibition of influenza virus infection by peptide-noble-metal nanoparticle conjugates. Beilstein J. Nanotechnol. 2019, 10, 1038–1047. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Hua, L.; Wang, H.; Xia, H.; Zhu, B. Silver Nanoparticle Based Codelivery of Oseltamivir to Inhibit the Activity of the H1N1 Influenza Virus through ROS-Mediated Signaling Pathways. ACS Appl. Mater. Interfaces 2016, 8, 24385–24393. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Guo, M.; Xu, T.; Wang, C.; Zhao, M.; Wang, H.; Chen, T.; Zhu, B. The inhibition of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Adv. 2017, 7, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Guzman, D.; Le Guen, P.; Villeret, B.; Sola, N.; Le Borgne, R.; Guyard, A.; Kemmel, A.; Crestani, B.; Sallenave, J.M.; Garcia-Verdugo, I. Silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosal immunity. Biomaterials 2019, 217, 119308. [Google Scholar] [CrossRef]
- Yang, X.X.; Huang, C.Z. Curcumin Modified Silver Nanoparticles for Highly Efficient Inhibition of Respiratory Syncytial Virus Infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef] [Green Version]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE 2014, 9, e104113. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Wegrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Dhanasezhian, A.; Srivani, S.; Govindaraju, K.; Parija, P.; Sasikala, S.; Kumar, M.R.R. Anti-Herpes Simplex Virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J. Geo Mar. Sci. 2019, 48, 1252–1257. [Google Scholar]
- Wan, C.; Tai, J.; Zhang, J.; Guo, Y.; Zhu, Q.; Ling, D.; Gu, F.; Gan, J.; Zhu, C.; Wang, Y.; et al. Silver nanoparticles selectively induce human oncogenic gamma-herpesvirus-related cancer cell death through reactivating viral lytic replication. Cell Death Dis. 2019, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- El-Mohamady, R.S.; Ghattas, T.A.; Zawrah, M.F.; Abd El-Hafeiz, Y.G.M. Inhibitory effect of silver nanoparticles on bovine herpesvirus-1. Int. J. Vet. Sci. Med. 2018, 6, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2020. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnology 2005, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Etemadzade, M.; Ghamarypour, A.; Zabihollahi, R.; Shabbak, G.; Shirazi, M.; Sahebjamee, H.; Vaziri, A.Z.; Assadi, A.; Ardestani, M.S.; Shandiz, S.A.S.; et al. Synthesis and evaluation of antiviral activities of novel sonochemical silver nanorods against HIV and HSV viruses. Asian Pac. J. Trop. Dis. 2016, 6, 854–858. [Google Scholar] [CrossRef]
- Kumar, S.D.; Singaravelu, G.; Ajithkumar, S.; Murugan, K.; Nicoletti, M.; Benelli, G. Mangrove-Mediated Green Synthesis of Silver Nanoparticles with High HIV-1 Reverse Transcriptase Inhibitory Potential. J. Clust. Sci. 2016, 28, 359–367. [Google Scholar] [CrossRef]
- Tsai, C.H.; Whiteley, C.G.; Lee, D.J. Interactions between HIV-1 protease, silver nanoparticles, and specific peptides. J. Taiwan Inst. Chem. Eng. 2019, 103, 20–32. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Tremiliosi, G.C.; Simoes, L.G.P.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.B.; Durigon, E.L.; Machado, R.R.G.; Medina, D.S.; Ribeiro, L.K.; Rosa, I.L.V.; et al. Ag nanoparticles-based antimicrobial polycotton fabrics to prevent the transmission and spread of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Rajawat, S.; Malik, M.M. Anticancer Activity of Green Silver Nanoparticles against He-La Cervical Cancer Cell Lines. Mater. Today Proc. 2019, 18, 841–847. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, Z.; Lui, V.C.H.; Wong, K.K.Y.; Tam, P.K.H.; Lee, P.; Lok, C.N.; Lamb, J.R.; Chen, Y.; Xia, H. Silver nanoparticle treatment ameliorates biliary atresia syndrome in rhesus rotavirus inoculated mice. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1041–1050. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.C.; Ogunleye, G.E.; Ogbole, O. Biomedical application of greenly synthesized silver nanoparticles using the filtrate of Trichoderma viride: Anticancer and immunomodulatory potentials. Polim. Med. 2019, 49, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Randazzo, W.; Fabra, M.J.; Lagaron, J.M.; Aznar, R.; Sánchez, G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT Food Sci. Technol. 2017, 79, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Sofy, A.R.; Hmed, A.A.; Abd El Haliem, N.F.; Zein, M.A.; Elshaarawy, R.F.M. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydr. Polym. 2019, 226, 115261. [Google Scholar] [CrossRef]
- Mane, P.C.; Chaudhari, R.D.; Shinde, M.D.; Kadam, D.D.; Song, C.K.; Amalnerkar, D.P.; Lee, H. Designing Ecofriendly Bionanocomposite Assembly with Improved Antimicrobial and Potent on-site Zika Virus Vector Larvicidal Activities with its Mode of Action. Sci. Rep. 2017, 7, 15531. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, S.; Tripathi, P. A facile and rapid method for green synthesis of Achyranthes aspera stem extract-mediated silver nano-composites with cidal potential against Aedes aegypti L. Saudi J. Biol. Sci. 2019, 26, 698–708. [Google Scholar] [CrossRef]
- Amarasinghe, L.D.; Wickramarachchi, P.; Aberathna, A.; Sithara, W.S.; De Silva, C.R. Comparative study on larvicidal activity of green synthesized silver nanoparticles and Annona glabra (Annonaceae) aqueous extract to control Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Heliyon 2020, 6, e04322. [Google Scholar] [CrossRef]
- Aziz, A.T.; Alshehri, M.A.; Alanazi, N.A.; Panneerselvam, C.; Trivedi, S.; Maggi, F.; Sut, S.; Dall’Acqua, S. Phytochemical analysis of Rhazya stricta extract and its use in fabrication of silver nanoparticles effective against mosquito vectors and microbial pathogens. Sci. Total Environ. 2020, 700, 134443. [Google Scholar] [CrossRef]
- Elumalai, D.; Hemavathi, M.; Deepaa, C.V.; Kaleena, P.K. Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors. Parasite Epidemiol. Control 2017, 2, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Sutthanont, N.; Attrapadung, S.; Nuchprayoon, S. Larvicidal Activity of Synthesized Silver Nanoparticles from Curcuma zedoaria Essential Oil against Culex quinquefasciatus. Insects 2019, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Azarudeen, R.M.S.T.; Govindarajan, M.; Amsath, A.; Muthukumaran, U.; Benelli, G. Single-Step Biofabrication of Silver Nanocrystals Using Naregamia alata: A Cost Effective and Eco-Friendly Control Tool in the Fight Against Malaria, Zika Virus and St. Louis Encephalitis Mosquito Vectors. J. Clust. Sci. 2016, 28, 179–203. [Google Scholar] [CrossRef] [Green Version]
- Jinu, U.; Rajakumaran, S.; Senthil-Nathan, S.; Geetha, N.; Venkatachalam, P. Potential larvicidal activity of silver nanohybrids synthesized using leaf extracts of Cleistanthus collinus (Roxb.) Benth. ex Hook.f. and Strychnos nux-vomica L. nux-vomica against dengue, Chikungunya and Zika vectors. Physiol. Mol. Plant Pathol. 2018, 101, 163–171. [Google Scholar] [CrossRef]
- Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology meets oncology: Nanomaterials in brain cancer research, diagnosis and therapy. Materials 2019, 12, 1588. [Google Scholar]
- Hulvat, M.C. Cancer Incidence and Trends. Surg. Clin. N. Am. 2020, 100, 469–481. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Feng, L.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 2018, 21, 55–73. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar]
- Gorbet, M.J.; Ranjan, A. Cancer immunotherapy with immunoadjuvants, nanoparticles, and checkpoint inhibitors: Recent progress and challenges in treatment and tracking response to immunotherapy. Pharmacol. Ther. 2020, 207, 107456. [Google Scholar] [CrossRef]
- Pothipor, C.; Wiriyakun, N.; Putnin, T.; Ngamaroonchote, A.; Jakmunee, J.; Ounnunkad, K.; Laocharoensuk, R.; Aroonyadet, N. Highly sensitive biosensor based on graphene–poly (3-aminobenzoic acid) modified electrodes and porous-hollowed-silver-gold nanoparticle labelling for prostate cancer detection. Sens. Actuators B Chem. 2019, 296, 126657. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Feyziazar, M.; Solhi, E.; Mokhtarzadeh, A.; Soleymani, J.; Shadjou, N.; Jouyban, A.; Mahboob, S. Ultrasensitive immunoassay of breast cancer type 1 susceptibility protein (BRCA1) using poly (dopamine-beta cyclodextrine-Cetyl trimethylammonium bromide) doped with silver nanoparticles: A new platform in early stage diagnosis of breast cancer and efficient management. Microchem. J. 2019, 145, 778–783. [Google Scholar] [CrossRef]
- Nigam Joshi, P.; Agawane, S.; Athalye, M.C.; Jadhav, V.; Sarkar, D.; Prakash, R. Multifunctional inulin tethered silver-graphene quantum dots nanotheranostic module for pancreatic cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Karuppaiah, A.; Siram, K.; Selvaraj, D.; Ramasamy, M.; Babu, D.; Sankar, V. Synergistic and enhanced anticancer effect of a facile surface modified non-cytotoxic silver nanoparticle conjugated with gemcitabine in metastatic breast cancer cells. Mater. Today Commun. 2020, 23, 100884. [Google Scholar] [CrossRef]
- Brkic Ahmed, L.; Milic, M.; Pongrac, I.M.; Marjanovic, A.M.; Mlinaric, H.; Pavicic, I.; Gajovic, S.; Vinkovic Vrcek, I. Impact of surface functionalization on the uptake mechanism and toxicity effects of silver nanoparticles in HepG2 cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 107, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Azhar, N.A.; Ghozali, S.Z.; Abu Bakar, S.A.; Lim, V.; Ahmad, N.H. Suppressing growth, migration, and invasion of human hepatocellular carcinoma HepG2 cells by Catharanthus roseussilver nanoparticles. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2020, 67, 104910. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Deng, N. Silver Nanoparticles Induce HePG-2 Cells Apoptosis Through ROS-Mediated Signaling Pathways. Nanoscale Res. Lett. 2016, 11, 198. [Google Scholar] [CrossRef] [Green Version]
- Bin-Jumah, M.; Al-Abdan, M.; Albasher, G.; Alarifi, S. Effects of Green Silver Nanoparticles on Apoptosis and Oxidative Stress in Normal and Cancerous Human Hepatic Cells in vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef] [Green Version]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhradnia, S.; Nystrom, B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Ishida, T. Anticancer activities of silver ions in cancer and tumor cells and DNA damages by Ag—DNA base-pairs reactions. MOJ Tumor Res. 2017. [Google Scholar] [CrossRef]
- Kanipandian, N.; Li, D.; Kannan, S. Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using Silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol. Rep. 2019, 23, e00339. [Google Scholar] [CrossRef]
- Lee, H.A.; Castro-Aceituno, V.; Abbai, R.; Moon, S.S.; Kim, Y.J.; Simu, S.Y.; Yang, D.C. Rhizome of Anemarrhena asphodeloides as mediators of the eco-friendly synthesis of silver and gold spherical, face-centred cubic nanocrystals and its anti-migratory and cytotoxic potential in normal and cancer cell lines. Artif. CellsNanomed. Biotechnol. 2018, 46, 285–294. [Google Scholar]
- Castro-Aceituno, V.; Abbai, R.; Moon, S.S.; Ahn, S.; Mathiyalagan, R.; Kim, Y.J.; Kim, Y.J.; Yang, D.C. Pleuropterus multiflorus (Hasuo) mediated straightforward eco-friendly synthesis of silver, gold nanoparticles and evaluation of their anti-cancer activity on A549 lung cancer cell line. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 93, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Castro-Aceituno, V.; Abbai, R.; Lee, H.A.; Simu, S.Y.; Han, Y.; Hurh, J.; Kim, Y.J.; Yang, D.C. Caspase-3/MAPK pathways as main regulators of the apoptotic effect of the phyto-mediated synthesized silver nanoparticle from dried stem of Eleutherococcus senticosus in human cancer cells. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 99, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 739–749. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver nanoparticles: Synthetic routes, in vitro toxicity and theranostic applications for cancer disease. Nanomaterials 2018, 8, 319. [Google Scholar]
- Ahn, E.Y.; Jin, H.; Park, Y. Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 204–216. [Google Scholar] [CrossRef]
- Majeed, S.; Aripin, F.H.B.; Shoeb, N.S.B.; Danish, M.; Ibrahim, M.N.M.; Hashim, R. Bioengineered silver nanoparticles capped with bovine serum albumin and its anticancer and apoptotic activity against breast, bone and intestinal colon cancer cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 254–263. [Google Scholar] [CrossRef]
- Gomathi, A.C.; Xavier Rajarathinam, S.R.; Mohammed Sadiq, A.; Rajeshkumar, S. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug Deliv. Sci. Technol. 2020, 55, 101376. [Google Scholar] [CrossRef]
- Al-kawmani, A.A.; Alanazi, K.M.; Farah, M.A.; Ali, M.A.; Hailan, W.A.Q.; Al-Hemaid, F.M.A. Apoptosis-inducing potential of biosynthesized silver nanoparticles in breast cancer cells. J. King Saud Univ. Sci. 2020, 32, 2480–2488. [Google Scholar] [CrossRef]
- Almalki, M.A.; Khalifa, A.Y.Z. Silver nanoparticles synthesis from Bacillus sp KFU36 and its anticancer effect in breast cancer MCF-7 cells via induction of apoptotic mechanism. J. Photochem. Photobiol. B Biol. 2020, 204, 111786. [Google Scholar] [CrossRef]
- Hepokur, C.; Kariper, I.A.; Misir, S.; Ay, E.; Tunoglu, S.; Ersez, M.S.; Zeybek, U.; Kuruca, S.E.; Yaylim, I. Silver nanoparticle/capecitabine for breast cancer cell treatment. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 61, 104600. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Saquib, Q.; Wahab, R.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg. Chem. Appl. 2018, 2018, 9390784. [Google Scholar] [PubMed] [Green Version]
- Yuan, Y.G.; Zhang, S.; Hwang, J.Y.; Kong, I.K. Silver Nanoparticles Potentiates Cytotoxicity and Apoptotic Potential of Camptothecin in Human Cervical Cancer Cells. Oxidative Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef]
- Algebaly, A.S.; Mohammed, A.E.; Abutaha, N.; Elobeid, M.M. Biogenic Synthesis of Silver Nanoparticles: Antibacterial and Cytotoxic Potential. Saudi J. Biol. Sci. 2019, 27, 1340–1351. [Google Scholar]
- Korkmaz, N.; Ceylan, Y.; Hamid, A.; Karadağ, A.; Bülbül, A.S.; Aftab, M.N.; Çevik, Ö.; Şen, F. Biogenic silver nanoparticles synthesized via Mimusops elengi fruit extract, a study on antibiofilm, antibacterial, and anticancer activities. J. Drug Deliv. Sci. Technol. 2020, 59, 101864. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Al-Brahim, J.S.; Mohammed, A.E. Antioxidant, cytotoxic and antibacterial potential of biosynthesized nanoparticles using bee honey from two different floral sources in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 363–373. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Zheng, Y.; Wang, Z.; Ma, Z.; Yang, Q.; Yao, B.; Zhao, Y.; Zhang, H. A green approach of synthesizing of silver nanoparticles and their antibacterial and cytotoxicity activities. New J. Chem. 2018. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Tasharrofi, N.; Saber, M.M. Green synthesis of silver nanoparticles using Teucrium polium leaf extract and assessment of their antitumor effects against MNK45 human gastric cancer cell line. J. Mol. Struct. 2020, 1208, 127889. [Google Scholar] [CrossRef]
- Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A.; Amiri, O.; Goli, H.R.; Rafiei, A.; Kardan, M.; Salavati-Niasari, M. Facile green synthesis and characterization of Crataegus microphylla extract-capped silver nanoparticles (CME@Ag-NPs) and its potential antibacterial and anticancer activities against AGS and MCF-7 human cancer cells. J. Alloys Compd. 2020, 820, 153186. [Google Scholar] [CrossRef]
- Ferreira, L.A.B.; Garcia-Fossa, F.; Radaic, A.; Duran, N.; Favaro, W.J.; de Jesus, M.B. Biogenic silver nanoparticles: In vitro and in vivo antitumor activity in bladder cancer. Eur. J. Pharm. Biopharm. 2020, 151, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Danagoudar, A.; Pratap, G.K.; Shantaram, M.; Ghosh, K.; Kanade, S.R.; Joshi, C.G. Characterization, cytotoxic and antioxidant potential of silver nanoparticles biosynthesised using endophytic fungus (Penicillium citrinum CGJ-C1). Mater. Today Commun. 2020, 25, 101385. [Google Scholar] [CrossRef]
- Danagoudar, A.; Pratap, G.K.; Shantaram, M.; Chatterjee, B.; Ghosh, K.; Kanade, S.R.; Joshi, C.G. Cancer cell specific cytotoxic potential of the silver nanoparticles synthesized using the endophytic fungus, Penicillium citrinum CGJ-C2. Mater. Today Commun. 2020, 25, 101442. [Google Scholar] [CrossRef]
- Sattari, R.; Khayati, G.R.; Hoshyar, R. Biosynthesis and characterization of silver nanoparticles capped by biomolecules by fumaria parviflora extract as green approach and evaluation of their cytotoxicity against human breast cancer MDA-MB-468 cell lines. Mater. Chem. Phys. 2020, 241, 122438. [Google Scholar] [CrossRef]
- Aygun, A.; Gulbagca, F.; Nas, M.S.; Alma, M.H.; Calimli, M.H.; Ustaoglu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Cellat, K.; Sen, F. Biological synthesis of silver nanoparticles using Rheum ribes and evaluation of their anticarcinogenic and antimicrobial potential: A novel approach in phytonanotechnology. J. Pharm. Biomed. Anal. 2020, 179, 113012. [Google Scholar] [CrossRef]
- Nesrin, K.; Yusuf, C.; Ahmet, K.; Ali, S.B.; Muhammad, N.A.; Suna, S.; Fatih, S. Biogenic silver nanoparticles synthesized from Rhododendron ponticum and their antibacterial, antibiofilm and cytotoxic activities. J. Pharm. Biomed. Anal. 2020, 179, 112993. [Google Scholar] [CrossRef]
- Dinparvar, S.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Aydogdu, M.; Aktas, D. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. J. Photochem. Photobiol. B Biol. 2020, 208, 111902. [Google Scholar] [CrossRef]
- Karimzadeh, K.; Elham, S.; Bakhshi, N.; Ramzanpoor, M. Biogenic silver nanoparticles using Oxalis corniculata characterization and their clinical implications. J. Drug Deliv. Sci. Technol. 2019, 54, 101263. [Google Scholar] [CrossRef]
- Deepika, S.; Selvaraj, C.I.; Roopan, S.M. Screening bioactivities of Caesalpinia pulcherrima L. swartz and cytotoxicity of extract synthesized silver nanoparticles on HCT116cell line. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110279. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Bilal Khan Niazi, M.; Zubair, M.; Almatroudi, A.; Khurshid, M.; Tariq, F.; Mumtaz, R.; Li, B. Bioprospecting a native silver-resistant Bacillus safensis strain for green synthesis and subsequent antibacterial and anticancer activities of silver nanoparticles. J. Adv. Res. 2020, 24, 475–483. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Kong, L.; Wu, T.; Zhang, T.; Tang, M.; et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020, 256, 113430. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Zahrudin, A.H.B.; Dash, G.K. Biosynthesis and characterization of silver nanoparticles from fungal species and its antibacterial and anticancer effect. Karbala Int. J. Mod. Sci. 2018, 4, 86–92. [Google Scholar]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2020, 202, 111642. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Krishnamoorthy, V.; Jagadeesan, Y.; Vilwanathan, R.; Balaiah, A. Annona muricata assisted biogenic synthesis of silver nanoparticles regulates cell cycle arrest in NSCLC cell lines. Bioorg. Chem. 2020, 95, 103451. [Google Scholar] [CrossRef] [PubMed]
- Varunkumar, K.; Anusha, C.; Saranya, T.; Ramalingam, V.; Raja, S.; Ravikumar, V. Avicennia marina engineered nanoparticles induce apoptosis in adenocarcinoma lung cancer cell line through p53 mediated signaling pathways. Process Biochem. 2020, 94, 349–358. [Google Scholar] [CrossRef]
- Majeed, S.; Bakhtiar, N.F.B.; Danish, M.; Mohamad Ibrahim, M.N.; Hashim, R. Green approach for the biosynthesis of silver nanoparticles and its antibacterial and antitumor effect against osteoblast MG-63 and breast MCF-7 cancer cell lines. Sustain. Chem. Pharm. 2019, 12, 100138. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Q.; Dai, T.; Shao, J.; Wu, X.; Jiang, Z.; Jacob, J.A.; Jiang, C. Identification of possible reductants in the aqueous leaf extract of mangrove plant Rhizophora apiculata for the fabrication and cytotoxicity of silver nanoparticles against human osteosarcoma MG-63 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111252. [Google Scholar] [CrossRef]
- Francis, S.; Nair, K.M.; Paul, N.; Koshy, E.P.; Mathew, B. Green synthesized metal nanoparticles as a selective inhibitor of human osteosarcoma and pathogenic microorganisms. Mater. Today Chem. 2019, 13, 128–138. [Google Scholar] [CrossRef]
- Khan, T.; Yasmin, A.; Townley, H.E. An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids Surf. B Biointerfaces 2020, 194, 111156. [Google Scholar] [CrossRef]
- Cameron, S.J.; Hosseinian, F.; Willmore, W.G. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef] [Green Version]
- Yesilot, S.; Aydin, C. Silver nanoparticles; a new hope in cancer therapy? East. J. Med. 2019, 24, 111–116. [Google Scholar]
- Yang, T.; Yao, Q.; Cao, F.; Liu, Q.; Liu, B.; Wang, X.H. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: Insight into the cytotoxicity and antiangiogenesis. Int. J. Nanomed. 2016, 11, 6679–6692. [Google Scholar] [CrossRef] [Green Version]
- Baghani, M.; Es-haghi, A. Characterization of silver nanoparticles biosynthesized using Amaranthus cruentus. Bioinspir. Biomim. Nanobiomater. 2020, 9, 129–136. [Google Scholar] [CrossRef]
- Ghandehari, S.; Tabrizi, M.; Ardalan, P. Evaluation of Anti-angiogenic Activity of Silver Nanoparticle Synthesis by Rubina tinctorum L (Ru-AgNPs) Using Chicken Chorioallantoic Membrane (CAM) Assay. J. Arak Univ. Med. Sci. 2018, 21, 82–90. [Google Scholar]
- Hao, M.; Kong, C.; Jiang, C.; Hou, R.; Zhao, X.; Li, J.; Wang, Y.; Gao, Y.; Zhang, H.; Yang, B.; et al. Polydopamine-coated Au-Ag nanoparticle-guided photothermal colorectal cancer therapy through multiple cell death pathways. Acta Biomater. 2019, 83, 414–424. [Google Scholar] [CrossRef]
- He, X.; Peng, C.; Qiang, S.; Xiong, L.H.; Zhao, Z.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Ma, N.; Tang, B.Z. Less is more: Silver-AIE core@shell nanoparticles for multimodality cancer imaging and synergistic therapy. Biomaterials 2020, 238, 119834. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Y.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. AgFe3O4C nanoparticles for multi-modal imaging-guided chemo-photothermal synergistic targeting for cancer therapy. Anal. Chim. Acta 2019, 1086, 122–132. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.H.; Jeong, J.H.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Silver nanoparticle-embedded graphene oxide-methotrexate for targeted cancer treatment. Colloids Surf. B Biointerfaces 2017, 153, 95–103. [Google Scholar] [CrossRef]
- Poudel, B.K.; Soe, Z.C.; Ruttala, H.B.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Gautam, M.; Ou, W.; Nguyen, H.T.; Jeong, J.H.; et al. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int. J. Pharm. 2018, 548, 92–103. [Google Scholar] [CrossRef]
- Sakr, T.M.; Khowessah, O.M.; Motaleb, M.A.; Abd El-Bary, A.; El-Kolaly, M.T.; Swidan, M.M. I-131 doping of silver nanoparticles platform for tumor theranosis guided drug delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 122, 239–245. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A.; Fekete, G.; Singh, T. Cytotoxic and radiosensitizing potential of silver nanoparticles against HepG-2 cells prepared by biosynthetic route using Picrasma quassioides leaf extract. J. Drug Deliv. Sci. Technol. 2020, 55, 101479. [Google Scholar] [CrossRef]
- Manaloto, E.; Gowen, A.A.; Lesniak, A.; He, Z.; Casey, A.; Cullen, P.J.; Curtin, J.F. Cold atmospheric plasma induces silver nanoparticle uptake, oxidative dissolution and enhanced cytotoxicity in glioblastoma multiforme cells. Arch. Biochem. Biophys. 2020, 689, 108462. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Eo, S.H.; Seo, S.Y.; Kim, S.J.; Hong, S.K.; Choi, C.S.; Lee, K.H. A potential mediator for photodynamic therapy based on silver nanoparticles functionalized with porphyrin. J. Photochem. Photobiol. A Chem. 2019, 377, 26–35. [Google Scholar] [CrossRef]
- Zurina, I.M.; Presniakova, V.S.; Butnaru, D.V.; Svistunov, A.A.; Timashev, P.S.; Rochev, Y.A. Tissue engineering using a combined cell sheet technology and scaffolding approach. Acta Biomater. 2020. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Camci-Unal, G. Unconventional Tissue Engineering Materials in Disguise. Trends Biotechnol. 2020, 38, 178–190. [Google Scholar] [CrossRef]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Bakopoulou, A.; Tsouknidas, A.; Prymak, O.; Papi, R.; Choli-Papadopoulou, T.; Epple, M.; Michailidis, N.; Koidis, P.; et al. Assessment of cytotoxicity and antibacterial effects of silver nanoparticle-doped titanium alloy surfaces. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, e220–e233. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Putra, N.E.; Tierolf, M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020, 107, 325–337. [Google Scholar] [CrossRef]
- Odatsu, T.; Kuroshima, S.; Sato, M.; Takase, K.; Valanezhad, A.; Naito, M.; Sawase, T. Antibacterial Properties of Nano-Ag Coating on Healing Abutment: An In Vitro and Clinical Study. Antibiotics 2020, 9, 347. [Google Scholar] [CrossRef]
- Zhang, C.; Lan, J.; Wang, S.; Han, S.; Yang, H.; Niu, Q.; Wang, J.; Wang, Q.; Xiang, Y.; Wu, Y.; et al. Silver nanowires on acid-alkali-treated titanium surface: Bacterial attachment and osteogenic activity. Ceram. Int. 2019, 45, 24528–24537. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Bushroa, A.R.; Nasiri-Tabrizi, B.; Baradaran, S.; Amiri, A.; Saber-Samandari, S.; Khanahmadi, S.; Zeimaran, E.; Basirun, W.J.; Kalaiselvam, K.; et al. Simultaneous enhanced antibacterial and osteoblast cytocompatibility performance of Ti6Al7Nb implant by nano-silver/graphene oxide decorated mixed oxide nanotube composite. Surf. Coat. Technol. 2019, 360, 181–195. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Zhang, J.Y.; Wang, Y.B.; Li, C.M.; Lu, Z.S.; Hu, X.F.; Xu, L.Q. Deposition of catechol-functionalized chitosan and silver nanoparticles on biomedical titanium surfaces for antibacterial application. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Aguilar, L.E.; Park, C.H.; Kim, C.S. UV Light Assisted Coating Method of Polyphenol Caffeic Acid and Mediated Immobilization of Metallic Silver Particles for Antibacterial Implant Surface Modification. Polymers 2019, 11, 1200. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Xiong, S.; Zhuo, S.; Liu, C.; Miao, J.; Liu, D.; Wang, H.; Zhang, Y.; Wang, C.; Liu, Y. Nanosilver/poly (dl-lactic-co-glycolic acid) on titanium implant surfaces for the enhancement of antibacterial properties and osteoinductivity. Int. J. Nanomed. 2019, 14, 1849–1863. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Abbasi, M. Hydroxyapatite mineralization on chitosan-tragacanth gum/silica@silver nanocomposites and their antibacterial activity evaluation. Int J. Biol. Macromol. 2020, 151, 909–923. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Khoshnava, S.M.; Pagan, E.; Chen, X. Co-incorporation of graphene oxide/silver nanoparticle into poly-L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110812. [Google Scholar] [CrossRef]
- Gouveia, Z.; Perinpanayagam, H.; Zhu, J. Development of Robust Chitosan–Silica Class II Hybrid Coatings with Antimicrobial Properties for Titanium Implants. Coatings 2020, 10, 534. [Google Scholar] [CrossRef]
- Salaie, R.N.; Besinis, A.; Le, H.; Tredwin, C.; Handy, R.D. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110210. [Google Scholar] [CrossRef]
- Estrada-Cabrera, E.; Torres-Ferrer, L.R.; Aztatzi-Aguilar, O.G.; De Vizcaya-Ruiz, A.; Meraz-Rios, M.A.; Zarate-Triviño, D.G.; Arizmendi-Morquecho, A.; de Luna Bugallo, A.; Prokhorov, E.; Luna-Barcenas, G. Chitosan-bioglass coatings on partially nanostructured anodized Ti-6Al-4V alloy for biomedical applications. Surf. Coat. Technol. 2019, 375, 468–476. [Google Scholar] [CrossRef]
- Yu, W.Z.; Zhang, Y.; Liu, X.; Xiang, Y.; Li, Z.; Wu, S. Synergistic antibacterial activity of multi components in lysozyme/chitosan/silver/hydroxyapatite hybrid coating. Mater. Des. 2018, 139, 351–362. [Google Scholar] [CrossRef]
- Mareci, D.; Trincă, L.C.; Căilean, D.; Souto, R.M. Corrosion resistance of ZrTi alloys with hydroxyapatite-zirconia-silver layer in simulated physiological solution containing proteins for biomaterial applications. Appl. Surf. Sci. 2016, 389, 1069–1075. [Google Scholar] [CrossRef]
- Trincă, L.C.; Mareci, D.; Souto, R.M.; Lozano-Gorrín, A.D.; Izquierdo, J.; Burtan, L.; Motrescu, I.; Vulpe, V.; Pavel, G.; Strungaru, S.; et al. Osseointegration evaluation of ZrTi alloys with hydroxyapatite-zirconia-silver layer in pig’s tibiae. Appl. Surf. Sci. 2019, 487, 127–137. [Google Scholar] [CrossRef]
- Patil, S.; Singh, N. Antibacterial silk fibroin scaffolds with green synthesized silver nanoparticles for osteoblast proliferation and human mesenchymal stem cell differentiation. Colloids Surf. B Biointerfaces 2019, 176, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wenhao, Z.; Zhang, T.; Yan, J.; Li, Q.; Xiong, P.; Li, Y.; Cheng, Y.; Zheng, Y. In vitro and in vivo evaluation of structurally-controlled silk fibroin coatings for orthopedic infection and in-situ osteogenesis. Acta Biomater. 2020. [Google Scholar] [CrossRef]
- Raho, R.; Nguyen, N.Y.; Zhang, N.; Jiang, W.; Sannino, A.; Liu, H.; Pollini, M.; Paladini, F. Photo-assisted green synthesis of silver doped silk fibroin/carboxymethyl cellulose nanocomposite hydrogels for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110219. [Google Scholar] [CrossRef]
- Baras, B.H.; Sun, J.; Melo, M.A.S.; Tay, F.R.; Oates, T.W.; Zhang, K.; Weir, M.D.; Xu, H.H.K. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 1479–1489. [Google Scholar] [CrossRef]
- Marques, L.; Martinez, G.; Guidelli, É.; Tamashiro, J.; Segato, R.; Payão, S.L.M.; Baffa, O.; Kinoshita, A. Performance on Bone Regeneration of a Silver Nanoparticle Delivery System Based on Natural Rubber Membrane NRL-AgNP. Coatings 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Suteewong, T.; Wongpreecha, J.; Polpanich, D.; Jangpatarapongsa, K.; Kaewsaneha, C.; Tangboriboonrat, P. PMMA particles coated with chitosan-silver nanoparticles as a dual antibacterial modifier for natural rubber latex films. Colloids Surf. B Biointerfaces 2019, 174, 544–552. [Google Scholar] [CrossRef]
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Chen, T.; Zhang, N.; Wei, Q.; Tian, J.; Wang, Y.; Ma, C.; Lu, Y. Hydroxyapatite/silver electrospun fibers for anti-infection and osteoinduction. J. Adv. Res. 2020, 21, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, W.; Xu, L.; Feng, P.; Zhang, Y.; Yang, W.; Shuai, C. Halloysite nanotubes loaded with nano silver for the sustained-release of antibacterial polymer nanocomposite scaffolds. J. Mater. Sci. Technol. 2020, 46, 237–247. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A.; Kumar, R.; Pruncu, C.I.; Yadav, A. Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique. Mater. Des. 2020, 195, 109026. [Google Scholar] [CrossRef]
- Kumar Saini, R.; Prasad Bagri, L.; Bajpai, A.K. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 177, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Paterson, T.E.; Shi, R.; Tian, J.; Harrison, C.J.; De Sousa Mendes, M.; Hatton, P.V.; Li, Z.; Ortega, I. Electrospun Scaffolds Containing Silver-Doped Hydroxyapatite with Antimicrobial Properties for Applications in Orthopedic and Dental Bone Surgery. J. Funct. Biomater. 2020, 11, 58. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Rani, N.A.M.; Shahitha, F. Carboxymethyl cellulose nanofibres impregnated with silver nanoparticles for tissue engineering applications. Mater. Today Proc. 2019, 16, 1715–1721. [Google Scholar] [CrossRef]
- Urena-Saborio, H.; Rodríguez, G.; Madrigal-Carballo, S.; Gunasekaran, S. Characterization and applications of silver nanoparticles-decorated electrospun nanofibers loaded with polyphenolic extract from rambutan (Nepelium lappaceum). Materialia 2020, 11, 100687. [Google Scholar] [CrossRef]
- Xu, J.; Xu, N.; Zhou, T.; Xiao, X.; Gao, B.; Fu, J.; Zhang, T. Polydopamine coatings embedded with silver nanoparticles on nanostructured titania for long-lasting antibacterial effect. Surf. Coat. Technol. 2017, 320, 608–613. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Liu, Y.; Liu, J.; He, W.; Dong, Y. Synthesis of silver @hydroxyapatite nanoparticles based biocomposite and their assessment for viability of Osseointegration for rabbit knee joint anterior cruciate ligament rehabilitation. J. Photochem. Photobiol. B Biol. 2020, 202, 111677. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Stoica, A.E.; Dima-Balcescu, M.S.; Chircov, C.; Gharbia, S.; Balta, C.; Rosu, M.; Herman, H.; Holban, A.M.; Ficai, A.; et al. Electrospun Polyethylene Terephthalate Nanofibers Loaded with Silver Nanoparticles: Novel Approach in Anti-Infective Therapy. J. Clin. Med. 2019, 8, 1039. [Google Scholar] [CrossRef] [Green Version]
- Ziabka, M.; Dziadek, M.; Menaszek, E. Biocompatibility of Poly(acrylonitrile-butadiene-styrene) Nanocomposites Modified with Silver Nanoparticles. Polymers 2018, 10, 1257. [Google Scholar] [CrossRef] [Green Version]
- Ziabka, M.; Dziadek, M. Long-Term Stability of Two Thermoplastic Polymers Modified with Silver Nanoparticles. Nanomaterials 2019, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Desagani, D.; Chandran, G.; Ghosh, N.N.; Karthikeyan, G.; Waigaonkar, S.; Ganguly, A. Biocompatible agarose-chitosan coated silver nanoparticle composite for soft tissue engineering applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 637–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Farrage, N.M.; Oraby, A.H.; Abdelrazek, E.M.M.; Atta, D. Synthesis, characterization of Ag@PANI core-shell nanostructures using solid state polymerization method. Biointerface Res. Appl. Chem. 2019, 9, 3934–3941. [Google Scholar] [CrossRef]
- Howaili, F.; Mashreghi, M.; Shahri, N.M.; Kompany, A.; Jalal, R. Development and evaluation of a novel beneficent antimicrobial bioscaffold based on animal waste-fish swim bladder (FSB) doped with silver nanoparticles. Environ. Res. 2020, 188, 109823. [Google Scholar] [CrossRef]
- Saleh, T.; Ahmed, E.; Yu, L.; Kwak, H.H.; Kang, B.J.; Park, K.M.; Choi, K.Y.; Kim, B.M.; Kang, K.S.; Woo, H.M. Characterization of silver nanoparticle-modified decellularized rat esophagus for esophageal tissue engineering: Structural properties and biocompatibility. J. Biosci. Bioeng. 2019, 128, 613–621. [Google Scholar] [CrossRef]
- Niu, X.; Wei, Y.; Liu, Q.; Yang, B.; Ma, N.; Li, Z.; Zhao, L.; Chen, W.; Huang, D. Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering. Eur. Polym. J. 2020, 134, 109861. [Google Scholar] [CrossRef]
- Ran, L.; Zou, Y.; Cheng, J.; Lu, F. Silver nanoparticles in situ synthesized by polysaccharides from Sanghuangporus sanghuang and composites with chitosan to prepare scaffolds for the regeneration of infected full-thickness skin defects. Int. J. Biol. Macromol. 2019, 125, 392–403. [Google Scholar] [CrossRef]
- Srivastava, C.M.; Purwar, R.; Gupta, A.P. Enhanced potential of biomimetic, silver nanoparticles functionalized Antheraea mylitta (tasar) silk fibroin nanofibrous mats for skin tissue engineering. Int. J. Biol. Macromol. 2019, 130, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Talodthaisong, C.; Boonta, W.; Thammawithan, S.; Patramanon, R.; Kamonsutthipaijit, N.; Hutchison, J.A.; Kulchat, S. Composite guar gum-silver nanoparticle hydrogels as self-healing, injectable, and antibacterial biomaterials. Mater. Today Commun. 2020, 24, 100992. [Google Scholar] [CrossRef]
- Pham, T.N.; Jiang, Y.S.; Su, C.F.; Jan, J.S. In situ formation of silver nanoparticles-contained gelatin-PEG-dopamine hydrogels via enzymatic cross-linking reaction for improved antibacterial activities. Int. J. Biol. Macromol. 2020, 146, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Ounkaew, A.; Kasemsiri, P.; Jetsrisuparb, K.; Uyama, H.; Hsu, Y.I.; Boonmars, T.; Artchayasawat, A.; Knijnenburg, J.T.N.; Chindaprasirt, P. Synthesis of nanocomposite hydrogel based carboxymethyl starch/polyvinyl alcohol/nanosilver for biomedical materials. Carbohydr. Polym. 2020, 248, 116767. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Gradisnik, L. Tissue Augmentation in Wound Healing: The Role of Endothelial and Epithelial Cells. Med. Arch. 2018, 72, 444–448. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A cellular perspective. Physiol. Rev. 2018, 99, 665–706. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef]
- Diegidio, P.; Hermiz, S.J.; Ortiz-Pujols, S.; Jones, S.W.; van Duin, D.; Weber, D.J.; Cairns, B.A.; Hultman, C.S. Even Better Than the Real Thing? Xenografting in Pediatric Patients with Scald Injury. Clin. Plast. Surg. 2017, 44, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, P.; Bishop, A.; Smerdon, G. Safety and efficacy of hyperbaric oxygen therapy in chronic wound management: Current evidence. Chronic Wound Care Manag. Res. 2015, 2, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, H.; Gurevich, M.; Tamir, E.; Keren, E.; Alexander, L.; Hayes, P. Topical oxygen therapy stimulates healing in difficult, chronic wounds: A tertiary centre experience. J. Wound Care 2018, 27, 426–433. [Google Scholar]
- Lima, R.V.K.S.; Coltro, P.S.; Farina JÚNior, J.A. Negative pressure therapy for the treatment of complex wounds. Rev. Colégio Bras. Cir. 2017, 44, 81–93. [Google Scholar] [CrossRef]
- Sexton, F.; Healy, D.; Keelan, S.; Alazzawi, M.; Naughton, P. A systematic review and meta-analysis comparing the effectiveness of negative-pressure wound therapy to standard therapy in the prevention of complications after vascular surgery. Int. J. Surg. 2020, 76, 94–100. [Google Scholar] [CrossRef]
- Ousey, K.; Cutting, K.F.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 124–130. [Google Scholar]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Milne, K.E.; Penn-Barwell, J.G. Classification and management of acute wounds and open fractures. Surgery 2020, 38, 143–149. [Google Scholar] [CrossRef]
- Liu, X.; Nielsen, L.H.; Klodzinska, S.N.; Nielsen, H.M.; Qu, H.; Christensen, L.P.; Rantanen, J.; Yang, M. Ciprofloxacin-loaded sodium alginate/poly (lactic-co-glycolic acid) electrospun fibrous mats for wound healing. Eur. J. Pharm. Biopharm. 2018, 123, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Tihminlioglu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to be Used in Wound Dressing Applications. J. Polym. Environ. 2020. [Google Scholar] [CrossRef]
- Radulescu, M.; Andronescu, E.; Dolete, G.; Popescu, R.C.; Fufa, O.; Chifiriuc, M.C.; Mogoanta, L.; Balseanu, T.A.; Mogosanu, G.D.; Grumezescu, A.M.; et al. Silver Nanocoatings for Reducing the Exogenous Microbial Colonization of Wound Dressings. Materials 2016, 9, 345. [Google Scholar] [CrossRef] [Green Version]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogosanu, G.D.; Balseanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoanta, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2019, 557, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qi, Z.; Pan, S.; Zheng, S.; Wang, H.; Chang, Y.; Li, H.; Xue, P.; Yang, X.; Fu, C. Preparation, characterization and evaluation of a new film based on chitosan, arginine and gold nanoparticle derivatives for wound-healing efficacy. RSC Adv. 2020, 10, 20886–20899. [Google Scholar] [CrossRef]
- Yapijakis, C. Hippocrates of Kos, the father of clinical medicine, and Asclepiades of Bithynia, the father of molecular medicine. Review. In Vivo 2009, 23, 507–514. [Google Scholar]
- Alexander, J.W. History of the Medical use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar]
- Nam, G.; Purushothaman, B.; Rangasamy, S.; Song, J.M. Investigating the versatility of multifunctional silver nanoparticles: Preparation and inspection of their potential as wound treatment agents. Int. Nano Lett. 2015, 6, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Nherera, L.; Trueman, P.; Roberts, C.; Berg, L. Cost-effectiveness Analysis of Silver Delivery Approaches in the Management of Partial-thickness Burns. Wounds Compend. Clin. Res. Pract. 2018, 30, 160–167. [Google Scholar]
- Chae, J.K.; Kim, J.H.; Kim, E.J.; Park, K. Values of a patient and observer scar assessment scale to evaluate the facial skin graft scar. Ann. Dermatol. 2016, 28, 615–623. [Google Scholar] [PubMed]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care-Friend or Foe: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2020, 231, 115696. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Jiang, X.; Welch, C.; Croley, T.R.; Wong, T.Y.; Chen, C.; Fan, S.; Chong, Y.; Li, R.; Ge, C. Bactericidal effects of silver nanoparticles on lactobacilli and the underlying mechanism. ACS Appl. Mater. Interfaces 2018, 10, 8443–8450. [Google Scholar] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar]
- Hassabo, A.G.; El-Naggar, M.E.; Mohamed, A.L.; Hebeish, A.A. Development of multifunctional modified cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide. Carbohydr. Polym. 2019, 210, 144–156. [Google Scholar] [CrossRef]
- Montagut, A.M.; Granados, A.; Ballesteros, A.; Pleixats, R.; Llagostera, M.; Cortés, P.; Sebastián, R.M.; Vallribera, A. Antibiotic protected silver nanoparticles for microbicidal cotton. Tetrahedron 2019, 75, 102–108. [Google Scholar] [CrossRef]
- Baygar, T.; Sarac, N.; Ugur, A.; Karaca, I.R. Antimicrobial characteristics and biocompatibility of the surgical sutures coated with biosynthesized silver nanoparticles. Bioorg. Chem. 2019, 86, 254–258. [Google Scholar] [CrossRef]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Ontong, J.C.; Wunnoo, S.; Paosen, S.; Munah, S.; Voravuthikunchai, S.P. Antibacterial-coated silk surgical sutures by ex situ deposition of silver nanoparticles synthesized with Eucalyptus camaldulensis eradicates infections. J. Microbiol. Methods 2020, 174, 105955. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, W.; Duan, P.; Chen, J.; Zhang, Y.; Fu, F.; Diao, H.; Liu, X. One-pot fabrication of durable antibacterial cotton fabric coated with silver nanoparticles via carboxymethyl chitosan as a binder and stabilizer. Carbohydr. Polym. 2019, 204, 42–49. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Ali, S.W.; Joshi, M.; Rajendran, S.; Das, A.; Alagirusamy, R. Leveraging antibacterial efficacy of silver loaded chitosan nanoparticles on layer-by-layer self-assembled coated cotton fabric. Int. J. Biol. Macromol. 2020, 162, 548–560. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.G.; López-Saucedo, J.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Antimicrobial silver-loaded polypropylene sutures modified by radiation-grafting. Eur. Polym. J. 2018, 100, 290–297. [Google Scholar] [CrossRef]
- Chen, J.; Fan, L.; Yang, C.; Wang, S.; Zhang, M.; Xu, J.; Luo, S. Facile synthesis of Ag nanoparticles-loaded chitosan antibacterial nanocomposite and its application in polypropylene. Int. J. Biol. Macromol. 2020, 161, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, G.; Graziani, G.; Baldini, N.; Maso, A.; Focarete, M.L.; Berni, M.; Biscarini, F.; Bianchi, M.; Gualandi, C. Nanodecoration of electrospun polymeric fibers with nanostructured silver coatings by ionized jet deposition for antibacterial tissues. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110998. [Google Scholar] [CrossRef]
- Ross, J.A.; Allan, N.; Olson, M.; Schatz, C.; Nation, P.N.; Gawaziuk, J.P.; Sethi, J.; Liu, S.; Logsetty, S. Comparison of the efficacy of silver-based antimicrobial burn dressings in a porcine model of burn wounds. Burns 2020. [Google Scholar] [CrossRef]
- Vasile, B.S.; Birca, A.C.; Musat, M.C.; Holban, A.M. Wound Dressings Coated with Silver Nanoparticles and Essential Oils for The Management of Wound Infections. Materials 2020, 13, 1682. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Tan, J.; Chen, C.; Sun, T.; Wang, S.; Zhang, M. Biofabrication of silver nanoparticles and its application for development of wound dressing system in nursing care for burn injuries in children. J. Drug Deliv. Sci. Technol. 2019, 54, 101236. [Google Scholar] [CrossRef]
- Mohamed, N.; Madian, N.G. Evaluation of the mechanical, physical and antimicrobial properties of chitosan thin films doped with greenly synthesized silver nanoparticles. Mater. Today Commun. 2020, 25, 101372. [Google Scholar] [CrossRef]
- Mohamed, N.; Madian, N.G. Enhancement of the dynamic mechanical properties of chitosan thin films by crosslinking with greenly synthesized silver nanoparticles. J. Mater. Res. Technol. 2020, 9, 12970–12975. [Google Scholar] [CrossRef]
- Hernandez-Rangel, A.; Silva-Bermudez, P.; Espana-Sanchez, B.L.; Luna-Hernandez, E.; Almaguer-Flores, A.; Ibarra, C.; Garcia-Perez, V.I.; Velasquillo, C.; Luna-Barcenas, G. Fabrication and in vitro behavior of dual-function chitosan/silver nanocomposites for potential wound dressing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 750–765. [Google Scholar] [CrossRef]
- Rahimi, M.; Ahmadi, R.; Kafil, H.S.; Shafiei-Irannejad, V. A novel bioactive quaternized chitosan and its silver-containing nanocomposites as a potent antimicrobial wound dressing: Structural and biological properties. Mater. Sci. Eng. C 2019, 101, 360–369. [Google Scholar]
- Ambrogi, V.; Pietrella, D.; Donnadio, A.; Latterini, L.; Di Michele, A.; Luffarelli, I.; Ricci, M. Biocompatible alginate silica supported silver nanoparticles composite films for wound dressing with antibiofilm activity. Mater. Sci. Eng. C 2020, 112, 110863. [Google Scholar]
- Tarusha, L.; Paoletti, S.; Travan, A.; Marsich, E. Alginate membranes loaded with hyaluronic acid and silver nanoparticles to foster tissue healing and to control bacterial contamination of non-healing wounds. J. Mater. Sci. Mater. Med. 2018, 29, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef]
- Nešović, K.; Janković, A.; Radetić, T.; Vukašinović-Sekulić, M.; Kojić, V.; Živković, L.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Chitosan-based hydrogel wound dressings with electrochemically incorporated silver nanoparticles—In vitro study. Eur. Polym. J. 2019, 121, 109257. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Feng, Z.; Peng, K.; Wei, A.; Wang, Y.; Tong, Z.; Cheng, B. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos. Part B Eng. 2020, 197, 108139. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.; Rannier, L.; Andrade, L.N.; Chaud, M.V.; da Silva, C.F.; Correa, C.B.; de Albuquerque Junior, R.L.C.; de Costa, L.P.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, A.; Farooq, M.A. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308. [Google Scholar] [CrossRef]
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.W.; Hahm, D.H. Lignin-mediated green synthesis of AgNPs in carrageenan matrix for wound dressing applications. Int. J. Biol. Macromol. 2020, 159, 859–869. [Google Scholar] [CrossRef]
- Alvarado-Gomez, E.; Martínez-Castañon, G.; Sanchez-Sanchez, R.; Ganem-Rondero, A.; Yacaman, M.J.; Martinez-Gutierrez, F. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Mater. Sci. Eng. C 2018, 92, 621–630. [Google Scholar]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Natarajan, D.; Kiran, M.S. Fabrication of juglone functionalized silver nanoparticle stabilized collagen scaffolds for pro-wound healing activities. Int. J. Biol. Macromol. 2019, 124, 1002–1015. [Google Scholar] [CrossRef]
- Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials 2020, 10, 844. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Maharajan, K.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Bacterial cellulose matrix with in situ impregnation of silver nanoparticles via catecholic redox chemistry for third degree burn wound healing. Carbohydr. Polym. 2020, 245, 116573. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Pansara, C.; Mishra, R.; Mehta, T.; Parikh, A.; Garg, S. Formulation of Chitosan Stabilized Silver Nanoparticle-Containing Wound Healing Film: In Vitro and In Vivo Characterization. J. Pharm. Sci. 2020, 109, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Ali Buabeid, M.; Arafa, E.A.; Hussain, I.; Li, L.; Murtaza, G. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Int. J. Pharm. 2019, 564, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, B.; Li, J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antibacterial effect and wound healing ability of silver nanoparticles incorporation into chitosan-based nanofibrous membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, Z.; Jiang, Q.; Zheng, Y.; Chen, Z.; Chen, X. Characterization and biological evaluation of a novel silver nanoparticle-loaded collagen-chitosan dressing. Regen. Biomater. 2020, 7, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preethi, G.U.; Unnikrishnan, B.S.; Sreekutty, J.; Archana, M.G.; Anupama, M.S.; Shiji, R.; Raveendran Pillai, K.; Joseph, M.M.; Syama, H.P.; Sreelekha, T.T. Semi-interpenetrating nanosilver doped polysaccharide hydrogel scaffolds for cutaneous wound healing. Int. J. Biol. Macromol. 2020, 142, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.B.; Thambusamy, S. Preparation of silver nanoparticles and riboflavin embedded electrospun polymer nanofibrous scaffolds for in vivo wound dressing application. Process Biochem. 2020, 88, 148–158. [Google Scholar] [CrossRef]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Skin wound healing acceleration by Ag nanoparticles embedded in PVA/PVP/Pectin/Mafenide acetate composite nanofibers. Polym. Test. 2019, 79, 106022. [Google Scholar] [CrossRef]
- Mohseni, M.; Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Abdi, S.; Moravvej, H.; Vossoughi, M. A comparative study of wound dressings loaded with silver sulfadiazine and silver nanoparticles: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 564, 350–358. [Google Scholar] [CrossRef]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Dewi, F.; Hinchliffe, R.J. Foot complications in patients with diabetes. Surgery 2020, 38, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Hajji, S.; Khedir, S.B.; Hamza-Mnif, I.; Hamdi, M.; Jedidi, I.; Kallel, R.; Boufi, S.; Nasri, M. Biomedical potential of chitosan-silver nanoparticles with special reference to antioxidant, antibacterial, hemolytic and in vivo cutaneous wound healing effects. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Vijayakumar, S.; Malaikozhundan, B.; Thangaraj, M.P.; Ekambaram, P.; Murugan, T.; Velusamy, P.; Anbu, P.; Vaseeharan, B. Chitosan-coated silver nanoparticles promoted antibacterial, antibiofilm, wound-healing of murine macrophages and antiproliferation of human breast cancer MCF 7 cells. Polym. Test. 2020, 90, 106675. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K.; et al. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110925. [Google Scholar] [CrossRef]
- Kuwabara, M.; Sato, Y.; Ishihara, M.; Takayama, T.; Nakamura, S.; Fukuda, K.; Murakami, K.; Yokoe, H.; Kiyosawa, T. Healing of Pseudomonas aeruginosa-infected wounds in diabetic db/db mice by weakly acidic hypochlorous acid cleansing and silver nanoparticle/chitin-nanofiber sheet covering. Wound Med. 2020, 28, 100183. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]

| Product Type | Product Trademark | Company | Note |
|---|---|---|---|
| Wound dressing | Acticoat™ | Smith & Nephew, Inc. (London, UK) | Flexible and nonadherent nanocrystalline silver dressing Provides sustained broad-spectrum bactericidal activity against over 150 strains |
| Allevyn™ Ag | Absorbent and flexible silicone foam dressing impregnated with SSD Provides sustained long-term bactericidal effects | ||
| Actisorb™ Silver | 3M+KCI (MN, USA) | Activated charcoal layer impregnated with silver Provides anti-bacterial barrier action and bactericidal activity | |
| Silvercel™ | Nonwoven pad of natural polysaccharides blend and nylon fibers impregnated with ionic silver Provides sustained long-term bactericidal and anti-biofilm effects | ||
| Tegaderm™ Alginate Ag | Absorbent soft-gelling alginate dressing impregnated with silver Provides sustained long-term broad-spectrum bactericidal effects | ||
| Maxorb® Extra Ag+ | Medline Industries, Inc. (IL, USA) | Blend fibers of natural polysaccharides impregnated with ionic silver Provides a sustained and long-term barrier against bacteria absorbed in wound exudates | |
| Opticell® Ag+ | Absorbent and flexible gelling fiber format impregnated with ionic silver Provides sustained long-term bactericidal activity | ||
| SilvaSorb™ Sheet | Super-absorbent hydrogel sheet impregnated with ionic silver Provides sustained long-term bactericidal activity | ||
| SilvaSorb™ Gel | Hydrogel ointment impregnated with ionic silver Provides sustained broad-spectrum antimicrobial action | ||
| Aquacell® Ag | ConvaTec Group (Deeside, UK) | Nonwoven inner pad impregnated with ionic silver Provides long-term broad-spectrum bactericidal and bacteriostatic effects | |
| PolyMem Silver™ | Ferris Mfg. Corp. (TX, USA) | Foam dressing impregnated with nanocrystalline silver Provides fungicidal and broad-spectrum bactericidal effects | |
| SilvrSTAT® | ABL Medical (UT, USA) | Hydrogel dressing ointment impregnated with metallic silver Provides antimicrobial action in first- and second-degree burns | |
| Catheter coating | Silverline® Drainage Catheters | Spiegelberg GmbH & Co. (Hamburg, Germany) | Radiopaque polyurethane or silicone catheters modified with silver Provides antimicrobial and anti-biofilm effects in the case of drainage in central nervous system structures |
| Covidien® Foley Catheter | Medtronic (London, UK) | Outer and inner silicone catheter and balloon coated with ionic silver hydrogel coating Provides substantial antimicrobial activity by consistent release of ionic silver | |
| SilverSoaker™ Catheter | Halyard Health, Inc., (GA, USA) | Outer and inner catheter coated with metallic silver (SilvaGard™) Provides antimicrobial and anti-biofilm effects | |
| Bardex® Catheter | C.R. Bard Inc., (NJ, USA) | Latex Foley catheter modified with Bard® hydrogel and Bactiguard® silver coating Provides antibacterial and anti-biofilm effects | |
| Endotracheal tube | Agento® Silver-coated Endotracheal Tube | C.R. Bard Inc., (NJ, USA) | Endotracheal tube modified with a hydrophilic polymer coating containing silver particles Provides microbiological efficiency against ventilator-associated pneumonia |
| Bacterial Strain | Proposed Systems | Effects | Refs. |
|---|---|---|---|
| Bacillus subtilis (B. subtilis) | AgNPs biosynthesized with petai (Parkia speciosa), fig tree (Ficus hispida), pomegranate (Punica granatum), Sida cordifolia and Platycodon grandiflorum extracts | Antibacterial effect due to size-related cytotoxicity and phytochemicals | [135,136,137,138,139] |
| AgNPs biosynthesized with coriander (Coriandrum sativum) leaf extract and AgNPs bioreduced by Actinomycetes strain | Bacterial death due to cellular uptake and Ag+-mediated DNA damage | [140,141] | |
| Enterococcus faecalis (E. faecalis) | AgNPs biosynthesized with night-blooming jasmine (Cestrum nocturnum) extract | Bacteriostatic and bactericidal effects exhibited for lower and higher AgNPs concentrations, respectively | [142] |
| AgNPs bioreduced by Fusarium semitectum strain | Strong antibacterial and anti-biofilm activity | [143,144] | |
| Klebsiella pneumoniae (K. pneumoniae) | AgNPs biosynthesized with butterfly pea (Clitoria ternatea) and mango (Mangifera indica) flower extracts and wild ginger (Alpinia nigra) fruit extract | Antibacterial effect due to size-related cytotoxicity and phytochemicals | [145,146,147] |
| AgNPs bioreduced by Nostoc Bahar M. cyanobacteria | Strong bactericidal effect due to imbalance in bacterial antioxidants and enzymes, fragmentation and degradation of bacterial proteins | [148] | |
| AgNPs bioreduced by Bifidobacterium bifidum strain | Antibacterial activity due to inhibitory effects on efflux pump genes | [149] | |
| PVP-capped AgNPs | Antibacterial effects due to membrane disruption and cytoplasmic protein leakage, anti-biofilm effects due to inhibitory activity on extracellular protein substances | [150] | |
| Pseudomonas aeruginosa (P. aeruginosa) | AgNPs biosynthesized with sesame (Sesamum indicum) oil, horse chestnut (Aesculus hippocastanum) and stonebreaker (Phyllanthus niruri) extracts | Bacterial death due to cellular uptake and size-related intracellular toxicity | [151,152,153] |
| AgNPs dendronized with cationic carbosilane dendrons and modified with PEG | Destabilization of outer membrane, degradation of peptidoglycan layer (in conjunction with endolysin) | [154] | |
| AgNPs biosynthesized with eyebright (Euphrasia officinalis) leaf extract | Strong antibacterial and anti-biofilm activity | [155] | |
| AgNPs biosynthesized with Lysiloma acapulcensis extract | Antibacterial effect due to size-related cytotoxicity and phytochemicals | [156] | |
| Salmonella enterica (S. enterica) | AgNPs biosynthesized with green tea (Camellia sinensis) and jackfruit (Artocarpus heterophyllus) extracts | Synergistic inhibitory and bactericidal effects due to size-related toxicity and phytochemicals | [157,158] |
| AgNPs capped with afzelin and quercitrin extracted from Crotolaria tetragona | Bacteriostatic and bactericidal effects, anti-biofilm activity due to alteration of membrane potential and efflux pumps and modification of bacterial surface hydrophobicity | [159] | |
| AgNPs bioreduced by Penicillium polonicum strain | Strong bactericidal activity due to membrane disruption and cytoplasmic protein leakage | [160] | |
| Staphylococcus epidermidis (S. epidermidis) | AgNPs biosynthesized with river bushwillow (Combretum erythrophyllum) leaf extract, grape (Vitis vinifera) fruit extract and Elytraria acaulis leaf extract | Bacterial death due to cellular uptake and size-related intracellular toxicity | [161,162,163] |
| AgNPs biosynthesized with tea tree (Melaleuca alternifolia) essential oil | Inhibitory and bactericidal effects due to membrane disruption and bacterial internalization, synergistic toxicity related to AgNPs size and tea tree essential oil | [164] | |
| Streptococcus mutans (S. mutans) | AgNPs biosynthesized with citrus (Citrus limetta) peel extract | Antibacterial effect due to size-related membrane permeability alteration and anti-biofilm activity | [165] |
| SiO2-coated AgNPs biosynthesized with green tea (Camellia sinensis) extract | Strong antibacterial and anti-biofilm activity | [166] | |
| Streptococcus pyogenes (S. pyogenes) | AgNPs biosynthesized with Dodonaea viscosa extract and AgNPs bioreduced by Saccharopolyspora hirsute strain | Antibacterial effect due to size-related cytotoxicity and phytochemicals | [167,168] |
| Malignant Cells | Proposed Systems | Effects | Refs. |
|---|---|---|---|
| Bladder carcinoma | AgNPs bioreduced by Fusarium oxysporum strain | Apoptosis induced by DNA damage, reduced cellular migration and proliferation, tumor regression | [263] |
| Breast adenocarcinoma | AgNPs bioreduced by Penicillium citrinum strain | Apoptosis induced by DNA damage | [264,265] |
| AgNPs biosynthesized with fineleaf fumitory (Fumaria parviflora), rhododendron (Rhododendron ponticum), rhubarb (Rheum ribes) and cumin (Cuminum cyminum) extracts | Cell death evidenced on distinctive tumor cell lines | [266,267,268,269] | |
| Colorectal cancer | AgNPs biosynthesized with creeping woodsorrel (Oxalis corniculata) leaf extract | Cell death induced by apoptotic and necrotic mechanisms | [270] |
| AgNPs biosynthesized with peacock (Caesalpinia pulcherrima) flower extract | Cell death induced by apoptosis and membrane damage | [271] | |
| Hepatocellular carcinoma | AgNPs bioreduced by Bacillus safensis strain | Cell death induced by apoptotic and necrotic mechanisms | [272] |
| PVP-stabilized AgNPs | Cell death induced by damage of cellular organelles (especially mitochondria) and oxidative stress, upregulation of mitochondrial proapoptotic proteins | [273] | |
| Laryngeal carcinoma | AgNPs bioreduced by Penicillium italicum strain | Cell death induced by ROS-mediated membrane damage and essential enzymes impairment | [274] |
| Lung adenocarcinoma | AgNPs bioreduced by Bacillus amyloliquefaciens strain | Cell death induced by ROS generation and damage of cellular organelles | [275] |
| AgNPs biosynthesized with soursop (Annona muricate) and mangrove (Avicennia marina) leaf extracts | Apoptosis induced by ROS generation, downregulation of antiapoptotic genes and upregulation of proapoptotic genes | [276,277] | |
| Osteosarcoma | AgNPs biosynthesized with cempedak (Artocarpus integer) and mangrove (Rhizophora apiculata) leaf extracts and noni (Morinda citrifolia) bark extract | Cell death evidenced on distinctive tumor cell lines, cell death induced by membrane damage and oxidative stress | [278,279,280] |
| Rhabdomyosarcoma | AgNPs bioreduced by Bacillus sp. strain | Cell death induced by ROS generation | [281] |
| Proposed Systems | In Vitro Effects | In Vivo Effects | Refs. |
|---|---|---|---|
| CS films embedded with CS-stabilized AgNPs | Antibacterial effects against E. coli | Better and faster wound healing rate, reduced local inflammation and enhanced angiogenesis | [413] |
| CS/sericin films conjugated with AgNPs and loaded with Moxifloxacin | Antibacterial effects against E. coli, P. aeruginosa, S. epidermidis, drug-sensitive and drug-resistant S. aureus | Rapid and enhanced repair of infected burn wounds accelerated wound healing, reduced local inflammation, improved collagen deposition and angiogenesis | [414] |
| CS/PEO nanofibrous membranes incorporated with AgNPs | Antibacterial effects against S. aureus | Bactericidal effects in infected wounds, faster wound healing rate, improved regeneration of epidermis and neovascularization | [415] |
| CS/KGM hydrogel embedded with AgNPs | Antibacterial effects against E. coli and S. aureus Good biocompatibility on fibroblasts | Enhanced repair of infected wounds, reduced inflammation by regulating local levels of proinflammatory and anti-inflammatory interleukins | [416] |
| Collagen/CS dressing loaded with AgNPs | Antibacterial effects against E. coli, P. aeruginosa and S. aureus | Faster wound healing rate, enhanced re-epithelialization, reduced local inflammation, downregulation of inflammatory cytokine and upregulation of growth factors | [417] |
| Galactoxyloglucan hydrogel scaffolds decorated with AgNPs | Antimicrobial effects against E. coli, S. aureus and C. albicans Enhanced cellular adhesion and proliferation of fibroblasts | Bactericidal effects in infected wounds, better and faster wound healing rate, improved collagen deposition and angiogenesis | [418] |
| PVA/β-cyclodextrin nanofibrous scaffolds loaded with AgNPs and riboflavin | Antibacterial effects against E. coli and S. aureusEnhanced cellular proliferation of epithelial cells | Enhanced wound contraction and re-epithelialization | [419] |
| PVA/PVP/pectin/MF nanofibers embedded with AgNPs | Antibacterial effects against E. coli, P. aeruginosa and S. aureus Enhanced cellular proliferation of fibroblasts | Faster healing rate and tissue regeneration | [420] |
| PCL/PVA nanofibrous scaffolds loaded with AgNPs | Antibacterial effects against S. aureus Good biocompatibility on fibroblasts | Improved wound closure, faster healing rate, reduced inflammation, promoted angiogenesis | [421] |
| PU/CA nanofibrous scaffolds incorporated with AgNPs-decorated GO and curcumin | Antibacterial effects against P. aeruginosa and S. aureus Enhanced cellular proliferation of fibroblasts | Improved neovascularization and collagen deposition, faster adnexal healing response, accelerated wound healing and advanced epidermis regeneration | [422] |
| PU foam dressings incorporated with AgNPs and asiaticoside | Antibacterial effects against B. subtilis, E. coli, P. aeruginosa and S. aureus Enhanced cellular proliferation of fibroblasts | Safe skin application, improved and accelerated wound healing | [423] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. https://doi.org/10.3390/nano10112318
Gherasim O, Puiu RA, Bîrcă AC, Burdușel A-C, Grumezescu AM. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials. 2020; 10(11):2318. https://doi.org/10.3390/nano10112318
Chicago/Turabian StyleGherasim, Oana, Rebecca Alexandra Puiu, Alexandra Cătălina Bîrcă, Alexandra-Cristina Burdușel, and Alexandru Mihai Grumezescu. 2020. "An Updated Review on Silver Nanoparticles in Biomedicine" Nanomaterials 10, no. 11: 2318. https://doi.org/10.3390/nano10112318
APA StyleGherasim, O., Puiu, R. A., Bîrcă, A. C., Burdușel, A. -C., & Grumezescu, A. M. (2020). An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials, 10(11), 2318. https://doi.org/10.3390/nano10112318