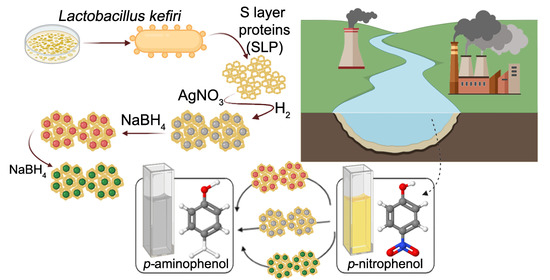
Synthesis and Catalytic Application of Silver Nanoparticles Supported on Lactobacillus kefiri S-Layer Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. S-Layer Protein Isolation
2.2. Synthesis of S-Layer Protein (SLP)-Supported Ag Nanoparticles
2.3. Characterization
2.4. Catalytic Test: p-Nitrophenol Reduction
2.5. Theoretical Calculations
3. Results and Discussion
3.1. Isolation and Characterization of the S-Layer Protein Supports
3.2. Synthesis and Characterization of Silver Nanoparticles (AgNPs)
3.3. Catalytic Test: p-Nitrophenol Reduction
3.4. Theoretical Calculations
4. Conclusions
- ✓
- Supported AgNPs were synthesized using supported S-layer proteins (SLPs) as bidimensional regularly arranged biotemplates. The nanoparticles were obtained in a simple way, without needing a stabilizer.
- ✓
- By different reduction strategies AgNPs of variable sizes were obtained on two different SLPs. A drastic reduction with NaBH4 led to large AgNPs (between 25 and 37 nm) whereas a smooth reduction with either H2 or H2/NaBH4 at low concentration, led to small AgNPs (sizes between 2 and 7 nm).
- ✓
- Conversion values between 75% and 80% of p-NP were observed for all the AgNPs tested, regardless of the average particle size of the NPs. Conversely, the apparent rate constant (Kapp) and TOF values were higher for Ag/S8R and Ag/S8G, the systems showing the smallest particle size.
- ✓
- Theoretical results confirmed the stretching of the N-O bond, meaning that it is activated for the hydrogenation reaction.
- ✓
- The most favored, thermodynamically stable adsorption mode of p-nitrophenolate species is through the nitro group, which would ensure p-aminophenol as the only feasible product of the reaction, which was corroborated experimentally.
- ✓
- Finally, obtaining AgNPs supported on a biological system such as LSP with outstanding catalytic activity is an interesting and environmentally friendly contribution, both with respect to its obtaining mechanism and also regarding the elimination of a dangerous pollutant such as p-NP.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kästner, C.; Thünemann, A.F. Catalytic Reduction of 4-Nitrophenol Using Silver Nanoparticles with Adjustable Activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Co-ord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Dong, X.Y.; Gao, Z.W.; Yang, K.F.; Zhang, W.Q.; Xu, L.W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Liao, G.; Zhao, W.; Li, Q.; Pang, Q.; Xu, Z. Novel Poly(acrylic acid)-modified Tourmaline/Silver Composites for Adsorption Removal of Cu(II) ions and Catalytic Reduction of Methylene Blue in Water. Chem. Lett. 2017, 46, 1631–1634. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Corma, A.; Concepción, P.; Boronat, M.; Sabater, M.J.; Navas, J.; Yacaman, M.J.; Larios, E.; Posadas, A.; López-Quintela, M.A.; Buceta, D.; et al. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nat. Chem. 2013, 5, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, H. Formation of metal nanowires on suspended single-walled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 3015–3017. [Google Scholar] [CrossRef]
- Peyser, L.A.; Roos, D.; Winterbourn, C.C. Photoactivated Fluorescence from Individual Silver Nanoclusters. Science 2001, 291, 103–106. [Google Scholar] [CrossRef]
- Brust, M.; Kiely, C.J.; Bethell, D.; Schiffrin, D.J. C60 Mediated Aggregation of Gold Nanoparticles. J. Am. Chem. Soc. 1998, 120, 12367–12368. [Google Scholar] [CrossRef]
- Okrut, A.; Runnebaum, R.C.; Ouyang, X.; Lu, J.; Aydin, C.; Hwang, S.-J.; Zhang, S.; Olatunji-Ojo, O.A.; Durkin, K.A.; Dixon, D.A.; et al. Selective molecular recognition by nanoscale environments in a supported iridium cluster catalyst. Nat. Nanotechnol. 2014, 9, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.A.; Buceta, D.; Blanco-Varela, C.; Mohamed, M.B.; Barone, G.; López-Quintela, M.A. Structure-Directing and High-Efficiency Photocatalytic Hydrogen Production by Ag Clusters. J. Am. Chem. Soc. 2014, 136, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Berti, L.; Alessandrini, A.; Facci, P. DNA-Templated Photoinduced Silver Deposition. J. Am. Chem. Soc. 2005, 127, 11216–11217. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.M.; Schultz, D.E.; Swasey, S.; Gwinn, E.G. Atomically Precise Arrays of Fluorescent Silver Clusters: A Modular Approach for Metal Cluster Photonics on DNA Nanostructures. ACS Nano 2015, 9, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Male, K.B.; Bouvrette, P.; Luong, J.H.T. Control of the Size and Distribution of Gold Nanoparticles by Unmodified Cyclodextrins. Chem. Mater. 2003, 15, 4172–4180. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Pérez-Juste, J.; Sánchez-Iglesias, A.; Giersig, M.; Liz-Marzán, L.M. Aligning Au Nanorods by Using Carbon Nanotubes as Templates. Angew. Chem. Int. Ed. 2005, 44, 4375–4378. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.C. Supported Metal Clusters: Synthesis, Structure, and Catalysis. Chem. Rev. 1995, 95, 511–522. [Google Scholar] [CrossRef]
- Petty, J.T.; Zheng, J.; Hud, A.N.V.; Dickson, R.M. DNA-Templated Ag Nanocluster Formation. J. Am. Chem. Soc. 2004, 126, 5207–5212. [Google Scholar] [CrossRef]
- O’Neill, P.R.; Young, K.; Schiffels, D.; Fygenson, D.K. Few-Atom Fluorescent Silver Clusters Assemble at Programmed Sites on DNA Nanotubes. Nano Lett. 2012, 12, 5464–5469. [Google Scholar] [CrossRef]
- Selva, J.; Martínez, S.E.; Buceta, D.; Rodríguez-Vázquez, M.J.; Blanco, M.C.; López-Quintela, M.A.; Egea, G. Silver Sub-nanoclusters Electrocatalyze Ethanol Oxidation and Provide Protection against Ethanol Toxicity in Cultured Mammalian Cells. J. Am. Chem. Soc. 2010, 132, 6947–6954. [Google Scholar] [CrossRef]
- Vilar-Vidal, N.; Blanco, M.C.; Loópez-Quintela, M.A.; Rivas, J.; Serra, C. Electrochemical Synthesis of Very Stable Photoluminescent Copper Clusters. J. Phys. Chem. C 2010, 114, 15924–15930. [Google Scholar] [CrossRef]
- Samanta, P.K.; Periyasamy, G.; Manna, A.K.; Pati, S.K. Computational studies on structural and optical properties of single-stranded DNA encapsulated silver/gold clusters. J. Mater. Chem. 2012, 22, 6774–6781. [Google Scholar] [CrossRef]
- Beveridge, T.J. Bacterial S-layers. Curr. Opin. Struct. Biol. 1994, 4, 204–212. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Schuster, B.; Egelseer, E.M.; Pum, D.; Horejs, C.-M.; Tscheliessnig, R.; Ilk, N. Nanobiotechnology with S-Layer Proteins as Building Blocks. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 103, pp. 277–352. [Google Scholar]
- Mertig, M.; Kirsch, R.; Pompe, W.; Engelhardt, H. Fabrication of highly oriented nanocluster arrays by biomolecular templating. Eur. Phys. J. D 1999, 9, 45–48. [Google Scholar] [CrossRef]
- Wahl, R.; Mertig, M.; Raff, J.; Selenska-Pobell, S.; Pompe, W. Electron-beam induced formation of highly ordered palladium and platinum nanoparticle arrays on the S layer of Bacillus sphaericus NCTC 9602. Adv. Mater. 2001, 13, 736–740. [Google Scholar] [CrossRef]
- Huggias, S.; Bolla, P.A.; Serradell, M.A.; Casella, M.; Peruzzo, P.J. Platinum Nanoparticles Obtained at Mild Conditions on S-Layer Protein/Polymer Particle Supports. Langmuir 2020, 36, 1201–1211. [Google Scholar] [CrossRef]
- Huggias, S.; Bolla, P.A.; Azcarate, J.C.; Serradell, M.A.; Casella, M.L.; Peruzzo, P.J. Noble metal nanoparticles-based heterogeneous bionano-catalysts supported on S-layer protein/polyurethane system. Catal. Today 2020. [Google Scholar] [CrossRef]
- Korkmaz, N.; Ostermann, K.; Rödel, G. Calcium dependent formation of tubular assemblies by recombinant S-layer proteins in vivo and in vitro. Nanotechnology 2011, 22, 095601. [Google Scholar] [CrossRef]
- Owoseni-Fagbenro, K.A.; Saifullah, S.; Imran, M.; Perveen, S.; Rao, K.; Fasina, T.M.; Olasupo, I.A.; Adams, L.A.; Ali, I.; Shah, M.R. Egg proteins stabilized green silver nanoparticles as delivery system for hesperidin enhanced bactericidal potential against resistant S. aureus. J. Drug Deliv. Sci. Technol. 2019, 50, 347–354. [Google Scholar] [CrossRef]
- Su-juan, Y.; Yong-guang, Y.; Jing-fu, L. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78. [Google Scholar]
- Zhang, Z.; Shao, C.; Sun, Y.; Mu, J.; Zhang, M.; Zhang, P.; Guo, Z.; Liang, P.; Wang, C.; Liu, Y. Tubular nanocomposite catalysts based on size-controlled and highly dispersed silver nanoparticles assembled on electrospun silicananotubes for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 1387–1395. [Google Scholar] [CrossRef]
- Dong, G.; Cao, Y.; Zheng, S.; Zhou, J.; Li, W.; Zaera, F.; Zhou, X. Catalyst consisting of Ag nanoparticles anchored on amine-derivatized mesoporous silica nanospheres for the selective hydrogenation of dimethyl oxalate to methyl glycolate. J. Catal. 2020, 391, 155–162. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Catalytic reduction of 4-nitrophenol by silver nanoparticles stabilized on environmentally benign macroscopic biopolymer hydrogel. Bioresour. Technol. 2013, 132, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Vo, N.T.; Kim, D. Highly robust magnetically recoverable Ag/Fe2O3 nanocatalyst for chemoselective hydrogenation of nitroarenes in water. Appl. Catal. A Gen. 2017, 538, 148–156. [Google Scholar] [CrossRef]
- Ferguson, G.A.; Mehmood, F.; Rankin, R.B.; Greeley, J.P.; Vajda, S.; Curtiss, L.A. Exploring Computational Design of Size-Specific Subnanometer Clusters Catalysts. Top. Catal. 2012, 55, 353–365. [Google Scholar] [CrossRef]
- Negreiros, F.R.; Aprà, E.; Barcaro, G.; Sementa, L.; Vajda, S.; Fortunelli, A. A first-principles theoretical approach to heterogeneous nanocatalysis. Nanoscale 2012, 4, 1208–1219. [Google Scholar] [CrossRef]
- Negreiros, F.R.; Barcaro, G.; Sementa, L.; Fortunelli, A. Concepts in theoretical heterogeneous ultrananocatalysis. Comptes Rendus Chim. 2014, 17, 625–633. [Google Scholar] [CrossRef]
- Sementa, L.; Barcaro, G.; Negreiros, F.R.; Fortunelli, A. Ligand/cluster/support catalytic complexes in heterogeneous ultrananocatalysis: NO oxidation on Ag3/MgO(100). Phys. Chem. Chem. Phys. 2014, 16, 26570–26577. [Google Scholar] [CrossRef] [Green Version]
- García Verga, L.; Skylaris, C.K. DFT modeling of metallic nanoparticles in Computational Modeling of Nanoparticles. In Self-Assembly of Nano- and Micro-Structured Materials Using Colloidal Engineering, 1st ed.; Bromley, S.T., Woodley, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 12, pp. 2–339. [Google Scholar]
- Bolla, P.A.; Sanz, A.; Huggias, S.; Ruggera, J.F.; Serradell, M.A.; Casella, M.L. Regular arrangement of Pt nanoparticles on S-layer proteins isolated from Lactobacillus kefiri: Synthesis and catalytic application. Mol. Catal. 2020, 481, 110262. [Google Scholar] [CrossRef]
- Carasi, P.; Trejo, F.M.; Pérez, P.F.; De Antoni, G.L.; Serradell, M.D.L.A. Surface proteins from Lactobacillus kefir antagonize in vitro cytotoxic effect of Clostridium difficile toxins. Anaerobe 2012, 18, 135–142. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobili, P.; Serradell, M.D.L.A.; Trejo, S.A.; Puigvert, F.X.A.; Abraham, A.G.; De Antoni, G.L. Heterogeneity of S-layer proteins from aggregating and non-aggregating Lactobacillus kefir strains. Antonie Leeuwenhoek 2009, 95, 363–372. [Google Scholar] [CrossRef]
- Malamud, M.; Carasi, P.; Bronsoms, S.; Trejo, S.A.; Serradell, M.D.L. Ángeles Lactobacillus kefiri shows inter-strain variations in the amino acid sequence of the S-layer proteins. Antonie Leeuwenhoek 2016, 110, 515–530. [Google Scholar] [CrossRef]
- Malamud, M.; Cavallero, G.; Casabuono, A.C.; Lepenies, B.; Serradell, M.D.L.A.; Couto, A.S. Immunostimulation by Lactobacillus kefiri S-layer proteins with distinct glycosylation patterns requires different lectin partners. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Cavallero, G.J.; Malamud, M.; Casabuono, A.C.; Serradell, M.D.L. Ángeles, S.M.; Couto, A.S. A glycoproteomic approach reveals that the S-layer glycoprotein of Lactobacillus kefiri CIDCA 83111 is O- and N-glycosylated. J. Proteom. 2017, 162, 20–29. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Buceta, D.; Rivas, J.; López-Quintela, M.A. From Nano- to Angstrom Technology. In Metal Nanoparticles and Clusters; Deepak, F.L., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–30. [Google Scholar]
- Herrera-Melián, J.; Martín-Rodríguez, A.; Ortega-Méndez, A.; Araña, J.; Doña-Rodríguez, J.; Pérez-Peña, J. Degradation and detoxification of 4-nitrophenol by advanced oxidation technologies and bench-scale constructed wetlands. J. Environ. Manag. 2012, 105, 53–60. [Google Scholar] [CrossRef]
- Lai, T.-L.; Yong, K.-F.; Yu, J.-W.; Chen, J.-H.; Shu, Y.-Y.; Wang, C.-B. High efficiency degradation of 4-nitrophenol by microwave-enhanced catalytic method. J. Hazard. Mater. 2011, 185, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Yoshimura, T.; Esumi, K. Preparation of Gold−Dendrimer Nanocomposites by Laser Irradiation and Their Catalytic Reduction of 4-Nitrophenol. Langmuir 2003, 19, 5517–5521. [Google Scholar] [CrossRef]
- Biella, S.; Porta, F.; Prati, L.; Rossi, M. Surfactant-Protected Gold Particles: New Challenge for Gold-on-Carbon Catalysts. Catal. Lett. 2003, 90, 23–29. [Google Scholar] [CrossRef]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thunemann, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Sahu, K.; Singh, J.; Mohapatra, S. Catalytic reduction of 4-nitrophenol and photocatalytic degradation of organic pollutants in water by copper oxide nanosheets. Opt. Mater. 2019, 93, 58–69. [Google Scholar] [CrossRef]
- Perry, D.A.; Cordova, J.S.; Smith, L.G.; Son, H.J.; Biris, A.S. Characterization of aminophenol isomer adsorption on silver nanostructures. Vib. Spectrosc. 2011, 55, 77–84. [Google Scholar] [CrossRef]







| Catalyst | Kapp (h−1) | ds (nm) * | D (%) | TOF (mol p-NP/mol Ag × h) |
|---|---|---|---|---|
| Ag/S1G | 16.8 | 4.79 | 24.41 | 11.0 |
| Ag/S1R | 13.7 | 6.79 | 17.21 | 8.9 |
| Ag/S1V | 5.4 | 25.24 | 4.37 | 3.3 |
| Ag/S8G | 17.7 | 3.5 | 33.36 | 11.2 |
| Ag/S8R | 18.47 | 2.65 | 44.10 | 12.0 |
| Ag/S8V | 8.1 | 36.88 | 3.22 | 5.2 |
| Crystalline Face | Substrate Species | Adsorption Mode | lO–Ag (A) | lO–N (A) | Eads (eV) |
|---|---|---|---|---|---|
| (111) | p-nitrophenol | perpendicular | 2.26 | 1.27 | −1.04 |
| flat | 2.29 | 1.29 | −1.09 | ||
| p-nitrophenolate | perpendicular | 2.27 | 1.28 | −1.20 | |
| flat | 2.29 | 1.29 | −1.11 | ||
| (100) | p-nitrophenol | perpendicular | 2.33 | 1.28 | −0.63 |
| flat | 2.44 | 1.27 | −0.74 | ||
| p-nitrophenolate | perpendicular | 2.34 | 1.30 | −0.91 | |
| flat | 2.43 | 1.28 | −0.69 | ||
| - | p-nitrophenol | - | - | 1.23 | - |
| - | p-nitrophenolate | - | - | 1.25 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolla, P.A.; Huggias, S.; Serradell, M.A.; Ruggera, J.F.; Casella, M.L. Synthesis and Catalytic Application of Silver Nanoparticles Supported on Lactobacillus kefiri S-Layer Proteins. Nanomaterials 2020, 10, 2322. https://doi.org/10.3390/nano10112322
Bolla PA, Huggias S, Serradell MA, Ruggera JF, Casella ML. Synthesis and Catalytic Application of Silver Nanoparticles Supported on Lactobacillus kefiri S-Layer Proteins. Nanomaterials. 2020; 10(11):2322. https://doi.org/10.3390/nano10112322
Chicago/Turabian StyleBolla, Patricia A., Sofía Huggias, María A. Serradell, José F. Ruggera, and Mónica L. Casella. 2020. "Synthesis and Catalytic Application of Silver Nanoparticles Supported on Lactobacillus kefiri S-Layer Proteins" Nanomaterials 10, no. 11: 2322. https://doi.org/10.3390/nano10112322
APA StyleBolla, P. A., Huggias, S., Serradell, M. A., Ruggera, J. F., & Casella, M. L. (2020). Synthesis and Catalytic Application of Silver Nanoparticles Supported on Lactobacillus kefiri S-Layer Proteins. Nanomaterials, 10(11), 2322. https://doi.org/10.3390/nano10112322