Impedimetric Detection of Femtomolar Levels of Interleukin 6, Interleukin 8, and Tumor Necrosis Factor Alpha Based on Thermally Modified Nanotubular Titanium Dioxide Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TiO2 Nanotubes Fabrication
2.3. Thermal Modification of TNT
2.4. Evaluation of the Open Circuit Potential (OCP) and Contact Angle of TNT
2.5. Functionalization of TNT Layers with Antibodies and IL-6, IL-8, TNFα Detection
3. Results and Discussion
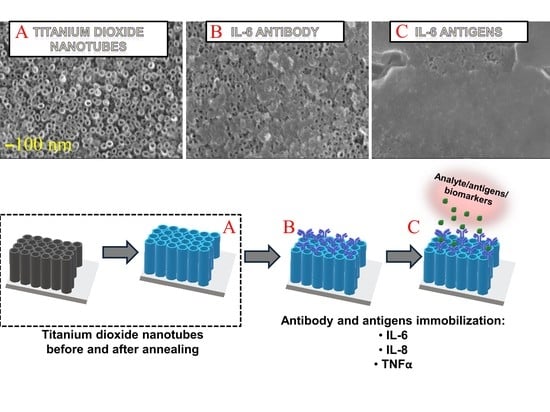
3.1. Characteristics of Titanium Dioxide Nanotubes
3.2. IL-6, IL-8, TNFα Antibodies Adsorption
3.3. Label-Free Detection of IL-6, IL-8, TNFα using Electrochemical Impedance Spectroscopy
3.4. Effect of pH of Electrolyte on IL-6, IL-8, TNFα Biosensor Response
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Marker conc. (pg/mL) | |Z| (Ω) | SD | RSD (%) | ReZ (Ω) | SD | RSD (%) | -ImZ (Ω) | SD | RSD (%) | -Zphase (o) | SD | RSD (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | ||||||||||||
| TNT/Ab | 5567 | 52 | 0.9 | 1100 | 53 | 4.8 | 5457 | 93 | 1.7 | 27 | 1.4 | 5.1 |
| 5 | 5459 | 86 | 1.6 | 960 | 68 | 7.1 | 5374 | 84 | 1.6 | 28 | 1.3 | 4.6 |
| 10 | 5618 | 83 | 1.5 | 954 | 74 | 7.8 | 5536 | 74 | 1.3 | 30 | 1.9 | 6.3 |
| 25 | 6684 | 64 | 1.0 | 1256 | 46 | 3.7 | 6667 | 65 | 1.0 | 32 | 1.7 | 5.3 |
| 50 | 6830 | 76 | 1.1 | 1333 | 36 | 2.7 | 6699 | 87 | 1.3 | 33 | 1.4 | 4.3 |
| 100 | 7047 | 75 | 1.1 | 1156 | 83 | 7.2 | 6952 | 84 | 1.2 | 36 | 1.8 | 5.0 |
| 250 | 7743 | 92 | 1.2 | 1593 | 109 | 6.8 | 7577 | 62 | 0.8 | 39 | 1.5 | 3.9 |
| 500 | 8203 | 108 | 1.3 | 1372 | 89 | 6.5 | 8087 | 85 | 1.1 | 39 | 1.4 | 3.6 |
| 1000 | 8490 | 120 | 1.4 | 1567 | 77 | 4.9 | 8345 | 58 | 0.7 | 42 | 1.9 | 4.5 |
| 2500 | 9372 | 184 | 2.0 | 1917 | 114 | 5.9 | 9174 | 137 | 1.5 | 44 | 1.4 | 3.2 |
| IL-8 | ||||||||||||
| TNT/Ab | 4507 | 43 | 1.0 | 822 | 57 | 6.9 | 4432 | 73 | 1.6 | 81 | 1.5 | 1.8 |
| 5 | 5831 | 69 | 1.2 | 1045 | 74 | 7.1 | 6159 | 58 | 0.9 | 79 | 1.9 | 2.4 |
| 10 | 6268 | 71 | 1.1 | 1018 | 56 | 5.5 | 6286 | 83 | 1.3 | 79 | 1.9 | 2.4 |
| 25 | 7051 | 98 | 1.4 | 1273 | 68 | 5.3 | 7253 | 59 | 0.8 | 78 | 1.7 | 2.2 |
| 50 | 7946 | 70 | 0.9 | 1535 | 82 | 5.3 | 7694 | 77 | 1.0 | 71 | 1.5 | 2.1 |
| 100 | 8217 | 94 | 1.1 | 2734 | 95 | 3.5 | 7253 | 85 | 1.2 | 71 | 1.7 | 2.4 |
| 150 | 8884 | 141 | 1.6 | 2881 | 85 | 2.9 | 8403 | 82 | 1.0 | 70 | 1.4 | 2.0 |
| 300 | 9669 | 109 | 1.1 | 3204 | 106 | 3.3 | 9123 | 95 | 1.0 | 69 | 1.9 | 2.7 |
| 600 | 11,839 | 118 | 1.0 | 5576 | 95 | 1.7 | 15,265 | 194 | 1.3 | 69 | 1.8 | 2.6 |
| 1200 | 16,252 | 168 | 1.0 | 9351 | 94 | 1.0 | 19,589 | 184 | 0.9 | 64 | 1.8 | 2.8 |
| 2500 | 21,706 | 182 | 0.8 | 14,594 | 134 | 0.9 | 21,499 | 192 | 0.9 | 56 | 1.8 | 3.2 |
| TNFα | ||||||||||||
| TNT/Ab | 4230 | 73 | 1.7 | 563 | 64 | 11.4 | 4193 | 65 | 1.6 | 82 | 1.8 | 2.2 |
| 5 | 4917 | 85 | 1.7 | 758 | 48 | 6.3 | 4802 | 59 | 1.2 | 79 | 2.1 | 2.7 |
| 10 | 6063 | 69 | 1.1 | 563 | 84 | 14.9 | 5724 | 128 | 2.2 | 80 | 1.9 | 2.4 |
| 25 | 7235 | 92 | 1.3 | 758 | 88 | 11.6 | 6842 | 95 | 1.4 | 80 | 1.8 | 2.2 |
| 50 | 7733 | 108 | 1.4 | 1185 | 92 | 7.8 | 7930 | 45 | 0.6 | 81 | 1.7 | 2.1 |
| 100 | 8253 | 125 | 1.5 | 1202 | 58 | 4.8 | 8157 | 184 | 2.3 | 81 | 2 | 2.5 |
| 250 | 9113 | 144 | 1.6 | 1256 | 93 | 7.4 | 7340 | 182 | 2.5 | 81 | 1.6 | 2.0 |
| 500 | 10,155 | 184 | 1.8 | 1883 | 183 | 9.7 | 9979 | 148 | 1.5 | 79 | 1.9 | 2.4 |
| 1000 | 12,081 | 199 | 1.6 | 2248 | 147 | 6.5 | 11,870 | 157 | 1.3 | 79 | 1.8 | 2.3 |
| 2500 | 18,082 | 176 | 1.0 | 2385 | 193 | 8.1 | 15,853 | 284 | 1.8 | 80 | 1.6 | 2.0 |
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, J.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Bohmwald, K.; Gálvez, M.S.; Canedo-Marroquín, G.; Pizarro-Ortega, M.S.; Andrade-Parra, C.; Gómez-Santander, F.; Kalergis, A.M. Contribution of Cytokines to Tissue Damage During Human Respiratory Syncytial Virus Infection. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Tilmann, J.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Justin, M.; Dominguez, C.; Hamilton, D.; Palena, K. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22–45. [Google Scholar]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu, A.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, P.; Jeyaraman, P.; Sachdeva, M.; Malhotra, P.; Ahluwalia, J. Synchronous hairy cell leukemia and chronic lymphocytic leukemia: A case report with a brief review of literature. Blood Res. 2018, 53, 160–181. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Brooks, E.; Li, Y.; Denny, P.; Ho, C.; Qi, F.; Shi, W.; Wolinsky, B.; Wu, L.; Wong, D.; et al. Detection of picomolar levels of interleukin-8 in human saliva by SPR. Lab. Chip. 2005, 5, 1017–1023. [Google Scholar] [CrossRef]
- Pedersen, L.M.; Schistad, E.; Jacobsen, L.M.; Røe, C.; Gjerstad, J. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: A 12-month prospective study. Brain Behav. Immun. 2015, 46, 132–136. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. Recent Advances in Electrochemical and Optical Biosensors Designed for Detection of Interleukin 6. Sensors 2020, 20, 646–673. [Google Scholar] [CrossRef] [Green Version]
- Berggren, C.; Bjarnason, B.; Johansson, G. An immunological Interleukine-6 capacitive biosensor using perturbation with a potentiostatic step. Biosens. Bioelectron. 1998, 13, 1061–1068. [Google Scholar] [CrossRef]
- German, A.; Messina, G.A.; Panini, V.; Martinez, A.; Raba, J. Microfluidic immunosensor design for the quantification of interleukin-6 in human serum samples. Anal. Biochem. 2008, 380, 262–267. [Google Scholar]
- Krishnamoorthy, S.; Iliadis, A.A.; Beic, T.; Chrousos, G.P. An interleukin-6 ZnO/SiO2/Si surface acoustic wave biosensor. Biosens. Bioelectron. 2008, 24, 313–318. [Google Scholar] [CrossRef]
- Kapoor, R.; Wang, C.W. Highly specific detection of interleukin-6 (IL-6) protein using combination tapered fiber-optic biosensor dip-probe. Biosens. Bioelectron. 2009, 24, 2696–2701. [Google Scholar] [CrossRef]
- Malhotra, R.; Patel, V.; Vaque, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplification. Anal. Chem. 2010, 82, 3118. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Huang, H.; Zhang, G.; Zhang, X.; Fang, B.; Wang, L. Dual Amplification Strategy for the Fabrication of Highly Sensitive Interleukin-6 Amperometric Immunosensor Based on Poly-Dopamine. Langmuir 2011, 27, 1224–1231. [Google Scholar] [CrossRef]
- Sardesai, P.; Barron, J.C.; Rusling, J.F. Carbon Nanotube Microwell Array for Sensitive Electrochemiluminescent Detection of Cancer Biomarker Proteins. Anal. Chem. 2011, 83, 6698–6703. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.K.; Vaze, A.; Rusling, J.F. Fabrication of immunosensor microwell arrays from gold compact discs for detection of cancer biomarker proteins. Lab Chip 2012, 12, 281–301. [Google Scholar] [CrossRef] [Green Version]
- Joo, J.; Kwon, D.; Yim, C.; Jeon, S. Highly Sensitive Diagnostic Assay for the Detection. ACS Nano 2012, 6, 4375–4381. [Google Scholar] [CrossRef]
- Wang, G.; He, X.; Chen, L.; Zhu, Y.; Zhang, X. Ultrasensitive IL-6 electrochemical immunosensor based on Au nanoparticles-graphene-silica biointerface. Colloids Surf. B 2014, 116, 714–719. [Google Scholar] [CrossRef]
- Chen, H.; Choo, T.K.; Huang, J.; Wang, Y.; Liu, Y.; Platt, M.; Tok, A.I.Y. Label-free electronic detection of interleukin-6 using horizontally aligned carbon nanotubes. Mater. Des. 2016, 90, 852–857. [Google Scholar] [CrossRef]
- Yang, T.; Wang, S.; Jin, H.; Bao, W.; Huang, S.; Wang, J. An electrochemical impedance sensor for the label-free ultrasensitive detection of interleukin-6 antigen. Sens. Actuators B Chem. 2013, 178, 310–315. [Google Scholar] [CrossRef]
- Sharma, R.; Deacon, S.E.; Nowak, D.; George, S.E.; Szymonik, M.P.; Tang, A.; Tomlinson, D.C.; Davies, A.G.; McPherson, M.J.; Wälti, C. Label-free electrochemical impedance biosensor to detect human interleukin-8 in serum with sub-pg/mL sensitivity. Biosen. Bioelectron. 2016, 80, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Winny, T. Optical Protein Sensor for Detecting Cancer Markers in Saliva. Biosens. Bioelectron. 2008, 24, 266–271. [Google Scholar]
- Hakim, M.M.A.; Lombardini, M.; Sun, K.; Giustiniano, F.; Roach, P.L.; Davies, D.E.; Ashburn, P. Thin Film Polycrystalline Silicon Nanowire Biosensors. Nano Lett. 2012, 12, 1868–1872. [Google Scholar] [CrossRef] [Green Version]
- Bosnjakovic, A.; Mishra, M.K.; Han, H.J.; Romero, R.; Kannan, R.M. A dendrimer-based immunosensor for improved capture and detection of tumor necrosis factor-α cytokine. Anal. Chim. Acta 2012, 720, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, Q.; Revzin, A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 2013, 138, 4321–4326. [Google Scholar] [CrossRef]
- Pui, T.S.; Bansal, T.; Kongsuphol, P.; Arya, S.K. Highly Sensitive Label Free Biosensor for Tumor Necrosis Factor. World Acad. Sci. Eng. Technol. 2012, 69, 747–750. [Google Scholar]
- Topkaya, S.; Azimzadeh, M.; Ozsoz, M. Electrochemical Biosensors for Cancer Biomarkers Detection: Recent Advances and Challenges. Electroanalysis 2016, 28, 1402–1419. [Google Scholar] [CrossRef]
- Arkusz, K.; Paradowska, E.; Nycz, M.; Krasicka-Cydzik, E. Influence of Thermal Modification and Morphology of TiO2 Nanotubes on Their Electrochemical Properties for Biosensors Applications. J. Nano Nanotech. 2018, 18, 3713–3721. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Arkusz, K.; Krasicka-Cydzik, E. The effect of phosphates and fluorides, included in TiO2 nanotube layers, on the performance of hydrogen peroxide detection. Archives Metall. Mat. 2018, 63, 765–772. [Google Scholar]
- Nycz, M.; Paradowska, E.; Arkusz, K.; Pijanowska, D.G. Influence of geometry and annealing temperature in argon atmosphere of TiO2 nanotubes on their electrochemical properties. Acta Bioeng. Biomech. 2020, 22, 165–177. [Google Scholar] [CrossRef]
- Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Paradowska, E.; Arkusz, K.; Pijanowska, D.G. The Influence of the Parameters of a Gold Nanoparticle Deposition Method on Titanium Dioxide Nanotubes, Their Electrochemical Response, and Protein Adsorption. Biosensors 2019, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Paradowska, E.; Arkusz, K.; Pijanowska, D.G. Comparison of Gold Nanoparticles Deposition Methods and Their Influence on Electrochemical and Adsorption Properties of Titanium Dioxide Nanotubes. Materials 2020, 13, 4269. [Google Scholar] [CrossRef]
- Arkusz, K. The Influence of Implantation on Mechanical Degradation of the Nanotubular Oxide Layer on Titanium Screws. Adv. Intell. Systems Comp. 2018, 1, 339–347. [Google Scholar]
- Arkusz, K.; Nycz, M.; Paradowska, E. Electrochemical Evaluation of the Compact and Nanotubular Oxide Layer Destruction under Ex Vivo Ti6Al4V ELI Transpedicular Screw Implantation. Materials 2020, 13, 176. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, A. Coadsorption of Horseradish Peroxidase with Thionine on TiO2Nanotubes for Biosensing. Langmuir 2005, 21, 8409–8413. [Google Scholar] [CrossRef]
- Kafi, A.K.M.; Wu, G.; Chen, A. A Novel Hydrogen Peroxide Biosensor Based on the Immobilization of Horseradish Peroxidase onto Au-modified Titanium Dioxide Nanotube Arrays. Biosens. Bioelectron. 2008, 24, 566–571. [Google Scholar] [CrossRef]
- Guo, C.; Hu, F.; Li, C.M.; Shen, P.K. Direct electrochemistry of hemoglobin on carbonized titania nanotubes and its application in a sensitive reagentless hydrogen peroxide biosensor. Biosens. Bioelectron. 2008, 24, 819–824. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Li, X.; Li, J. Biofunctionaltitania nanotubes for visible-light-activated photoelectrochemical biosensing. Anal. Chem. 2010, 82, 2253–2261. [Google Scholar] [CrossRef]
- Kafi, A.K.M.; Wu, G.; Benvenuto, P.; Chen, A. Highly sensitive amperometric H2O2 biosensor based on hemoglobin modified TiO2 nanotubes. J. Electroanal. Chem. 2011, 662, 64–69. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Y. Electrochemical biosensing based on polypyrrole/titania nanotube hybrid. Mat. Sci. Eng. C. 2013, 33, 5028–5035. [Google Scholar] [CrossRef]
- Hashemi, A.; Ezati, M.; Mohammadnejad, J.; Houshmand, B.; Faghihi, S. Chitosan Coating of TiO2 Nanotube Arrays for Improved Metformin Release and Osteoblast Differentiation. Int. J. Nanomed. 2020, 15, 4471–4481. [Google Scholar] [CrossRef]
- Abdolmajid, E.; Kharazi, H.; Chalaki, M.; Khojasteh, M.; Haghighat, S.; Attar, F.; Nemati, F.; Falahati, M. Titanium oxide nanoparticles fabrication, hemoglobin interaction, white blood cells cytotoxicity, and antibacterial studies. J. Biomol. Struct. Dyn. 2019, 37, 3007–3017. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, T.; Zhang, J. Photocatalytic degradation of methylene blue with spent FCC catalyst loaded with ferric oxide and titanium dioxide. Sci. Rep. 2020, 10, 12730–12737. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Wing Fen, Y. Recent Advances in Electrochemical and Optical Sensing of Dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, Y.; Jin, Q.; Fujishima, M.; Tada, H. Visible-light-activation of TiO2 nanotube array by the molecular iron oxide surface modification. Appl. Catal. B Environ. 2012, 19, 74–80. [Google Scholar] [CrossRef]
- García-Valverde, M.T.; Lucena, R.; Galán-Cano, F.; Cárdenas, S.; Valcárcel, M. Carbon coated titanium dioxide nanotubes: Synthesis, characterization and potential application as sorbents in dispersive micro solid phase extraction. J. Chromatogr. A 2014, 23, 26–32. [Google Scholar] [CrossRef]
- Mei, W.; Daojun, G.; Hulin, L. High activity of novel Pd/TiO2 nanotube catalysts for methanol electro-oxidation. J. Solid State Chem. 2005, 6, 1996–2000. [Google Scholar]
- Rong, Z.; Chen, F.; Jilin, J.; Yifeng, T. A C-reactive protein immunosensor based on platinum nanowire/titania nanotube composite sensitized electrochemiluminescence. Talanta 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Mandal, S.S.; Navratna, V.; Sharma, P.; Gopal, B.; Bhattacharyya, A.J. Titania nanotube-modified screen printed carbon electrodes enhance the sensitivity in the electrochemical detection of proteins. Bioelectrochemisty 2014, 98, 46–53. [Google Scholar] [CrossRef]
- Sun, S.; He, H.; Yang, C. Effects of Ca2+ and fulvic acids on atrazine degradation by nano-TiO2: Performances and mechanisms. Sci. Rep. 2019, 9, 8880. [Google Scholar] [CrossRef] [Green Version]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [Green Version]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Yan, J.; Zhang, Y. Stability of titania nanotube arrays in aqueous environment and the related factors. Sci. Rep. 2016, 6, 23065. [Google Scholar] [CrossRef] [Green Version]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Inter. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar]
- Kulkarni, M.; Flašker, A.; Lokar, M.; Mrak-Poljšak, K.; Mazare, A.; Artenjak, A.; Čučnik, S.; Kralj, S.; Velikonja, A.; Schmuki, P.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 18, 1359–7133. [Google Scholar]
- Reverberi, R.; Reverberi, L. Factor affecting the antygen-antibody reaction. Blood Transfus. 2007, 5, 227–240. [Google Scholar]
- Ward, L.D.; Zhang, J.G.; Checkey, G.; Preston, B.; Simpson, R.J. Effect of pH and denaturants on the folding and stability of murine interleukin-6. Protein Sci. 1993, 2, 1291–1300. [Google Scholar] [CrossRef] [Green Version]







| (wt.) | Non-Annealed | Annealed in Argon |
|---|---|---|
| Ti (%) | 65.9 ± 4.2 | 66.1 ± 3.1 |
| O (%) | 25.6 ± 2.6 | 33.9 ± 1.5 |
| F (%) | 8.6 ± 3.5 | - |
| IL-6 (pg/mL) | χ2 | Rs (ῼ) | R1 (ῼ) | CPE2 (Mho) |
|---|---|---|---|---|
| 0 | 0.025 | 33.5 | 427.4 | 7.54 × 10−9 |
| 5 | 0.077 | 39.8 | 319.7 | 5.22 × 10−9 |
| 10 | 0.022 | 54.2 | 49.9 | 4.46 × 10−9 |
| 25 | 0.049 | 57.8 | 13.9 | 2.37 × 10−6 |
| 50 | 0.016 | 68.1 | 2.5 | 3.43 × 10−4 |
| 100 | 0.043 | 80.6 | 11.5 | 2.35 × 10−4 |
| 250 | 0.092 | 100.9 | 189.2 | 2.25 × 10−4 |
| 500 | 0.094 | 110.6 | 1.10 × 1012 | 1.80 × 10−3 |
| 1000 | 0.050 | 117.1 | 1.10 × 1012 | 0.67 |
| 2500 | 0.040 | 140.4 | 1.10 × 1012 | 4.71 |
| IL-8 (pg/mL) | χ2 | R1 (ῼ) | CPE1 (Mho) × 10−4 | α1 |
| 0 | 0.025 | 54.8 | 3.1 | 0.439 |
| 5 | 0.020 | 83.6 | 2.8 | 0.772 |
| 10 | 0.026 | 88.4 | 2.7 | 0.783 |
| 25 | 0.097 | 110.9 | 1.7 | 0.797 |
| 50 | 0.044 | 113.8 | 1.3 | 0.820 |
| 100 | 0.026 | 118.4 | 1.2 | 0.827 |
| 150 | 0.087 | 308.1 | 0.14 | 0.834 |
| 300 | 0.093 | 332.7 | 0.15 | 0.838 |
| 600 | 0.041 | 474.9 | 0.0086 | 0.863 |
| 1200 | 0.016 | 631.1 | 0.0088 | 0.885 |
| 2500 | 0.046 | 27,553 | 0.00026 | 0.974 |
| TNFα (pg/mL) | χ2 | Rs (ῼ) | R2 (ῼ) | α1 |
| 0 | 0.056 | 28.1 | 3.18 | 0.765 |
| 5 | 0.019 | 55.9 | 70.4 | 0.780 |
| 10 | 0.094 | 73.6 | 72.8 | 0.792 |
| 25 | 0.053 | 91.9 | 91.3 | 0.837 |
| 50 | 0.026 | 101.9 | 103.1 | 0.860 |
| 100 | 0.035 | 108.6 | 152.0 | 0.863 |
| 250 | 0.015 | 123.4 | 181.4 | 0.871 |
| 500 | 0.064 | 140.5 | 247.4 | 0.899 |
| 1000 | 0.014 | 196.4 | 8511.0 | 0.980 |
| 2500 | 0.047 | 272.4 | 17,332.0 | 0.987 |
| Target | Composition of Sensitive Film | Detection Method | Limit of Detection | Linear Range | Ref. |
|---|---|---|---|---|---|
| IL-6 | Au rods/Ab1 | Potentiometric | 10 ng/mL | - | [10] |
| APCPG/CC/Ab/Ag/Ab1-Strepravidin-alkaline | Amperometric | 0.41 pg/mL | 0 ÷ 400 pg/mL | [11] | |
| ZnO/SiO2/Si-Ab | SAW | - | 20 ng/mL÷ 2 µg/mL | [12] | |
| Combination tapered fiber-optic biosensor (CTFOB)/Ab | Optic | 0.12 ng/mL | 40 pg/mL÷100 ng/mL | [13] | |
| SWCT-Ab1/Ag/Ab2-HRP | Amperometric | 0.05 pg/mL | 0.5÷5 pg/mL | [14] | |
| ITO/AuNP/PDOP Poly-dopamine -Ab-Ag-Ab1-HRP/Ab2/CNT | Amperometric | 1 pg/mL | 4÷800 pg/mL | [15] | |
| SWCNTforests-Ab1/Ag/Ab2-Ru(bpy)32+-silica | ECL | 0.25 pg/mL | - | [16] | |
| Au-Ab1/Ag/Ab2-Poly-HRP | Amperometric | 10 fg/mL | 10÷1300 fg/mL | [17] | |
| Magnetic-photocatalytic hybrid nanoparticles with a highly crystalline TiO2 shell-Ab | Microresonators | 0.1 pg/mL | - | [18] | |
| SiO2/Si/ CNT/AuNP-Ab | Impedimetric | 0.01 fg/mL | 0.01÷100 fg /ml | [19] | |
| SWCNT-Ab | FET | 1.37 pg/mL | - | [20] | |
| CNT/AuNP-Ab | Impedimetric | 0.01 fg/mL | 0.00001÷0.1 pg/mL | [21] | |
| IL-8 | PEG/Au/non-antibody capture proteinbased on a cystatin scaffold | Impedimetric | 90 fg/mL | 900 fg/mL÷900 ng/mL | [22] |
| ELISA surface with capture probe | Optical | 32 fg/mL | 5 fg/mL÷1 pg/mL | [23] | |
| Polysilicon nanowire-Ab | - | 80 fg/mL | - | [24] | |
| TNFα | Dendrimer-based PEG/Ab/Ag/Ab2-HRP | Optical | 0.48 pg/mL | 0 ÷ 300 pg/mL | [25] |
| MB labeled TNFα aptamer | Optical | 10 ng/mL | 10 ÷ 100 ng/mL | [26] | |
| Au-Si-dithiobis-succinimidyl propionate | Impedimetric | 1 pg/mL | 1 pg/mL÷1 ng/mL | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkusz, K.; Paradowska, E. Impedimetric Detection of Femtomolar Levels of Interleukin 6, Interleukin 8, and Tumor Necrosis Factor Alpha Based on Thermally Modified Nanotubular Titanium Dioxide Arrays. Nanomaterials 2020, 10, 2399. https://doi.org/10.3390/nano10122399
Arkusz K, Paradowska E. Impedimetric Detection of Femtomolar Levels of Interleukin 6, Interleukin 8, and Tumor Necrosis Factor Alpha Based on Thermally Modified Nanotubular Titanium Dioxide Arrays. Nanomaterials. 2020; 10(12):2399. https://doi.org/10.3390/nano10122399
Chicago/Turabian StyleArkusz, Katarzyna, and Ewa Paradowska. 2020. "Impedimetric Detection of Femtomolar Levels of Interleukin 6, Interleukin 8, and Tumor Necrosis Factor Alpha Based on Thermally Modified Nanotubular Titanium Dioxide Arrays" Nanomaterials 10, no. 12: 2399. https://doi.org/10.3390/nano10122399
APA StyleArkusz, K., & Paradowska, E. (2020). Impedimetric Detection of Femtomolar Levels of Interleukin 6, Interleukin 8, and Tumor Necrosis Factor Alpha Based on Thermally Modified Nanotubular Titanium Dioxide Arrays. Nanomaterials, 10(12), 2399. https://doi.org/10.3390/nano10122399