Role of 2‒13C Isotopic Glyphosate Adsorption on Silver Nanoparticles Based on Ninhydrin Reaction: A Study Based on Surface—Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of Silver Nanoparticles
2.4. Preparation of Solution
2.5. Preparation of Purple Color Dye (PD) Product
2.6. Theoretical Method
3. Results and Discussion
3.1. Characterization of the Ag Nanoparticles (NPs)
3.2. Molecular Geometry
3.3. DFT Calculations of 13–GLP and 12–GLP
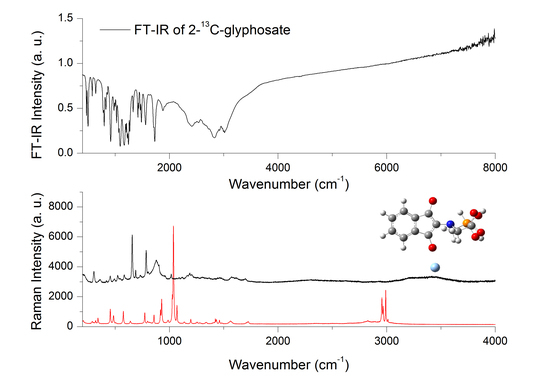
3.4. Experimental Raman Spectra of 13–GLP and 12–GLP
3.5. Experimental FT–IR Spectra of 13–GLP and 12–GLP
3.6. Raman and SERS Spectra of PD POroduct from 12–GLP/13–GLP
3.7. Quantitative Analysis of 13–GLP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tu, Q.; Yang, T.; Qu, Y.; Gao, S.; Zhang, Z.; Zhang, Q.; Wang, Y.; Wang, J.; He, L. In situ colorimetric detection of glyphosate on plant tissues using cysteamine-modified gold nanoparticles. Analyst 2019, 144, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Gao, Y.; Li, Y.; Li, X.; Zhang, H.; Han, X.X.; Zhao, B.; Su, L. Indirect glyphosate detection based on ninhydrin reaction and surface‒enhanced Raman scattering spectroscopy. Spectrochim. Acta A 2018, 195, 78–82. [Google Scholar] [CrossRef]
- Markus, A.M.; Kraus, M.; Miltner, A.; Hamer, U.; Novak, K.M. Effect of temperature, pH and total organic carbon variations on microbial turnover of 13C315N‒glyphosate in agricultural soil. Sci. Total Environ. 2019, 658, 697–707. [Google Scholar]
- Chan, Y.; Fei, W.; Sae, J.C.; Jun, Y.; Ruth, E.B. Phosphate oxygen isotope evidence for methylphosphonate sources of methane and dissolved inorganic phosphate. Sci. Total Environ. 2018, 644, 747–753. [Google Scholar]
- Feis, A.; Gelini, C.; Ricci, M.; Tognaccini, L.; Becucci, M.; Smulevich, G. Surface‒enhanced Raman scattering of glyphosate on dispersed silver nanoparticles: A reinterpretation based on model molecules. Vib. Spectrosc. 2020, 108, 103061. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface–enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Becke, A. Density–functional exchange–energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A. Density–functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle–Salvetti correlation–energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision A.02); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bulat, F.A.; Toro-Labbe, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative analysis of molecular surfaces: Areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, M.-L.; Xiong, J. Raman and SERS spectra of thiamethoxam and the Ag3–thiamethoxam complex: An experimental and theoretical investigation. J. Environ. Sci. Health B 2019, 54, 665–675. [Google Scholar] [CrossRef]
- Rocicler, O.; Holanda, C.B.S.; Daniel, L.M.V.; Paulo, T.C.F. High pressure Raman spectra and DFT calculation of glyphosate. Spectrochim. Acta. A 2020, 242, 118745–118752. [Google Scholar]
- Yael, J.A.; Fuhr, J.D.; Bocan, G.A.; Millone, A.D.; Tognalli, N.; Afonso, M.S.; Martiarena, M.L. Abiotic Degradation of Glyphosate into Aminomethylphosphonic Acid in the Presence of Metals. J. Agric. Food Chem. 2014, 62, 9651–9656. [Google Scholar] [CrossRef]
- Parameswari, A.; Asath, R.M.; Premkumar, R.; Benial, A.M.F. SERS and quantum chemical studies on N–methylglycine molecule on silver nanoparticles. J. Mol. Struct. 2017, 1138, 102–109. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Ando, R.A.; Sant’Ana, A.C.; Corio, P. Surface–enhanced Raman spectroscopy studies of organophosphorous model molecules and pesticides. Phys. Chem. Chem. Phys. 2012, 14, 15645–15651. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef] [PubMed]





| Theoretical Vibrations Frequency (After Scaling) | Experimental Raman (Solid State) | Experimental IR (Solid State) | Vibrational Assignments | |||
|---|---|---|---|---|---|---|
| 12–GLP | 13–GLP | 12–GLP | 13–GLP | 12–GLP | 13–GLP | |
| 532 | 531 | 511 | 504 | 501 | 501 | δ(O12H) + δ(C7H2) |
| 629 | 629 | 648 | 639 | 648 | 642 | τ(C2N5H) + τ(C7N5H) |
| 674 | 673 | ‒ | ‒ | ‒ | ‒ | ω(C10O12H) |
| 829 | 829 | 864 | 857 | 864 | 856 | νs(PO) + ν(PC) |
| 856 | 849 | 918 | 917 | 916 | 916 | C2N5C7C10 skel. |
| 890 | 886 | 933 | 928 | ‒ | ‒ | ρ(C7H2) |
| 979 | 979 | 979 | ‒ | 982 | 980 | ρ(C2H2) |
| 989 | 979.5 | 993 | 987 | 1001 | 997 | ω(O15H) + ω(O17H) |
| 996 | 995 | ‒ | 1026 | 1032 | 1026 | δ(HOP) |
| 1036 | 1027 | 1037 | 1036 | 1082 | 1067 | νs(C2N6C7) |
| 1137 | 1130 | 1082 | 1069 | 1095 | 1094 | νas(C2N5C7) |
| 1310 | 1309 | 1340 | 1338 | 1335 | 1331 | δ(C10O12H) + ω(C7H2) |
| 1328 | 1327 | 1422 | 1425 | 1421 | 1420 | ω(C7H2) |
| 1362 | 1350 | 1432 | 1432 | 1433 | 1433 | ω(C2H2) + ν(C10C7) |
| 1426 | 1426 | 1465 | 1460 | 1470 | 1462 | δ(C7H2) |
| 1464 | 1460 | 1482 | 1482 | 1485 | 1483 | ω(NH2) + δ(C2H2) |
| 2799 | 2792 | ‒ | ‒ | 2409 | 2411 | ν(C7H8) |
| 3001 | 2992 | 3001 | 2991 | 3001 | 2922 | ν(C7H9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.-L.; Gao, Y.; Jin, J.; Xiong, J.-F.; Han, X.X.; Zhao, B. Role of 2‒13C Isotopic Glyphosate Adsorption on Silver Nanoparticles Based on Ninhydrin Reaction: A Study Based on Surface—Enhanced Raman Spectroscopy. Nanomaterials 2020, 10, 2539. https://doi.org/10.3390/nano10122539
Xu M-L, Gao Y, Jin J, Xiong J-F, Han XX, Zhao B. Role of 2‒13C Isotopic Glyphosate Adsorption on Silver Nanoparticles Based on Ninhydrin Reaction: A Study Based on Surface—Enhanced Raman Spectroscopy. Nanomaterials. 2020; 10(12):2539. https://doi.org/10.3390/nano10122539
Chicago/Turabian StyleXu, Meng-Lei, Yu Gao, Jing Jin, Jin-Feng Xiong, Xiao Xia Han, and Bing Zhao. 2020. "Role of 2‒13C Isotopic Glyphosate Adsorption on Silver Nanoparticles Based on Ninhydrin Reaction: A Study Based on Surface—Enhanced Raman Spectroscopy" Nanomaterials 10, no. 12: 2539. https://doi.org/10.3390/nano10122539
APA StyleXu, M. -L., Gao, Y., Jin, J., Xiong, J. -F., Han, X. X., & Zhao, B. (2020). Role of 2‒13C Isotopic Glyphosate Adsorption on Silver Nanoparticles Based on Ninhydrin Reaction: A Study Based on Surface—Enhanced Raman Spectroscopy. Nanomaterials, 10(12), 2539. https://doi.org/10.3390/nano10122539