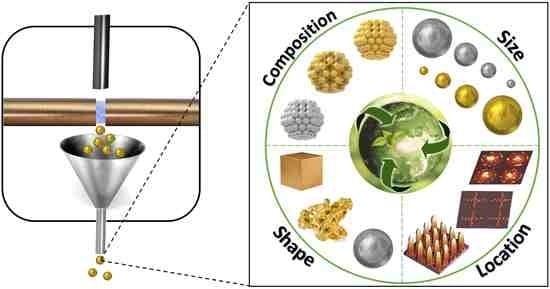
Green and Sustainable Manufacture of Ultrapure Engineered Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. SPNPs Nanomanufacture
2.2. Non-Agglomerated NPs Nanomanufacture
2.3. ENSs Nanomanufacture
2.4. Characterization
3. Results and Discussion
3.1. Engineering Composition
3.2. Engineering Size
3.3. Engineering Shape
3.4. Engineering Location
4. Conclusions
- ➢
- Higher sustainability
- A greener and more sustainable process to manufacture engineered nanomaterials (using no solvents, a lower gas flow rate, and reducing material waste)
- ➢
- Higher nanomanufacturability—scalability, reliability and commercial viability
- Industrially scalable process to manufacture engineered nanomaterials (gathering the best features ensuring a stable continuous production of tunable NPs)
- Low-cost process to manufacture engineered nanomaterials (avoiding expensive top-down or high-pressure equipment, raising affordability and guaranteeing commercial viability)
- Versatile process to manufacture engineered nanomaterials (from non-agglomerated NCs and nanoalloys, to 3D ENS)
- Reduced thermophoretic losses
- ➢
- Higher tunability of manufactured nanomaterials
- Control over agglomeration (enabling synthesis conditions with lower agglomeration, driving to the sustainable manufacture of SPNPs)
- Control over aggregation (allowing the production of smaller NCs)
- Control over the diffusion and mixture of atomic clusters while SPNPs are being formed, enabling a fine tuning of composition and/or improving compositional homogeneity
- Control over NCs availability during growth process (opening doors to shape control in the gas phase)
- Tunability over five nanoscale features of nanomaterials: degree of agglomeration, composition, size, shape, and location
- (1)
- Single-metal (Ag, Au) SPNPs as well as multi-metal (AuAg) nanoalloys with the narrowest composition distribution (ten times smaller than the gas-phase state of art) among non-solution approaches without pre-alloyed sources, being extensible to other compositions, including ceramic and composite NCs.
- (2)
- Au and Ag NPs measuring 3–20 nm with the lowest gas flow rate described for rod-to-rod SDGs (six times smaller than the state of art), which can be used as tunable building blocks for many applications, as well as the subsequent production of ENSs.
- (3)
- Amorphous, spherical and squared non-agglomerated NPs from 50 to 800 nm, the first shape-controlled ultrapure NPs obtained by a non-solution room-temperature gas-phase process, to the best of our knowledge.
- (4)
- Au and Ag ENSs, such as nanodisks, nanocrosses, and 3D nanopillars with enhanced stability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carenco, S.; Portehault, D.; Boissière, C.; Mézailles, N.; Sanchez, C. 25th Anniversary Article: Exploring Nanoscaled Matter from Speciation to Phase Diagrams: Metal Phosphide Nanoparticles as a Case of Study. Adv. Mater. 2014, 26, 371–390. [Google Scholar] [CrossRef]
- Pérez, J.; Bax, L.; Escolano, C. Roadmap Report on Nanoparticles; Willems & van den Wildenberg: Barcelona, Spain, 2005. [Google Scholar]
- Sebastian, V.; Arruebo, M.; Santamaria, J. Reaction Engineering Strategies for the Production of Inorganic Nanomaterials. Small 2014, 10, 835–853. [Google Scholar] [CrossRef]
- Barsotti, R.J., Jr.; Vahey, M.D.; Wartena, R.; Chiang, Y.M.; Voldman, J.; Stellacci, F. Assembly of Metal Nanoparticles into Nanogaps. Small 2007, 3, 488–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Loeffler, J.; Sutter, U.; Hedderich, R.; Fiedeler, U.; Ineke Malsch, G.T.; Koskinen, J.; Linder, M.; Lojkowski, W.; Moritz, T.; Zins, M.; et al. Overview on Promising Nanomaterials for Industrial Applications. Nanomaterial Roadmap 2015, FP6 nanoRoad sme. Karlsruhe, Germany. 2015. Available online: http://203.187.160.132:9011/www.tut.ee/c3pr90ntc0td/public/m/Mehaanikateaduskond/Instituudid/Materjalitehnika_instituut/MTX9100/Additional_reading/NanoMat2015.pdf (accessed on 17 January 2020).
- Liddle, J.A.; Gallatin, G.M. Nanomanufacturing: A Perspective. ACS Nano 2016, 10, 2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claridge, S.A.; Castleman, A.W., Jr.; Khanna, S.N.; Murray, C.B.; Sen, A.; Weiss, P.S. Cluster-Assembled Materials. ACS Nano 2009, 3, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Liu, G.; Zhou, Y.; Brown, K.A.; Chernyak, N.; Hedrick, J.L.; He, S.; Xie, Z.; Lin, Q.Y.; Dravid, V.P.; et al. Tip-Directed Synthesis of Multimetallic Nanoparticles. J. Am. Chem. Soc. 2015, 137, 9167–9173. [Google Scholar] [CrossRef]
- Türk, M.; Erkey, C. Synthesis of supported nanoparticles in supercritical fluids by supercritical fluid reactive deposition: Current state, further perspectives and needs. J. Supercrit. Fluid. 2018, 134, 176–183. [Google Scholar] [CrossRef]
- Schwyn, S.; Garwin, E.; Schmidt-Ott, A. Aerosol Generation by Spark Discharge. J. Aerosol Sci. 1988, 19, 639. [Google Scholar] [CrossRef] [Green Version]
- Watters, R.L.; DeVoe, J.R.; Shen, F.H.; Small, J.A.; Marinenko, R.B. Characteristics of Aerosols Produced by the Spark Discharge. Anal. Chem. 1989, 61, 1826–1833. [Google Scholar] [CrossRef]
- Meuller, B.O.; Messing, M.E.; Engberg, D.L.J.; Jansson, A.M.; Johansson, L.I.M.; Norlén, S.M.; Tureson, N.; Deppert, K. Review of Spark Discharge Generators for Production of Nanoparticle Aerosols. Aerosol Sci. Technol. 2012, 46, 1256–1270. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Ullman, M.; Vons, V.A.; Lafont, U.; Schmidt-Ott, A. Generation of Nanoparticles by Spark Discharge. J. Nanopart. Res. 2009, 11, 315. [Google Scholar] [CrossRef] [Green Version]
- Sengar, S.K.; Mehta, B.R. Govind. Size and Alloying Induced Changes in Lattice Constant, Core, and Valance Band Binding Energy in Pd-Ag, Pd, and Ag Nanoparticles: Effect of In-Flight Sintering Temperature. J. Appl. Phys. 2012, 112, 014307. [Google Scholar] [CrossRef]
- Hontañón, E.; Palomares, J.M.; Stein, M.; Guo, X.; Engeln, R.; Nirschl, H.; Kruis, F.E. The Transition from Spark to Arc Discharge and its Implications with Respect to Nanoparticle Production. J. Nanopart. Res. 2013, 15, 1957. [Google Scholar] [CrossRef]
- Efimov, A.A.; Lizunova, A.A.; Volkov, I.A.; Mylnikov, D.A.; Arsenov, P.V.; Ivanov, V.V. A New Approach to the High-Yield Synthesis of Nanoparticles by Spark Discharge. J. Phys. Conf. Ser. 2016, 741, 012035. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Kim, W.; Yu, J.; Lee, J.; Lee, H.; Woo, C.G.; Choi, M. A Study of Pin-to-Plate Type Spark Discharge Generator for Producing Unagglomerated Nanoaerosols. J. Aerosol. Sci. 2012, 52, 80–88. [Google Scholar] [CrossRef]
- Chae, S.; Lee, D.; Kim, M.-C.; Kim, D.S.; Choi, M. Wire-in-Hole-Type Spark Discharge Generator for Long-Time Consistent Generation of Unagglomerated Nanoparticles. Aerosol Sci. Technol. 2015, 49, 463–471. [Google Scholar] [CrossRef]
- Feng, J.; Huang, L.; Ludvigsson, L.; Messing, M.E.; Maisser, A.; Biskos, G.; Schmidt-Ott, A.A. General Approach to the Evolution of Singlet Nanoparticles from a Rapidly Quenched Point Source. J. Phys. Chem. C 2016, 120, 621–630. [Google Scholar] [CrossRef]
- Ahmad, S.; Laiho, P.; Zhang, Q.; Jiang, H.; Hussain, A.; Liao, Y.; Ding, E.-X.; Wei, N.; Kauppinen, E.I. Gas phase synthesis of metallic and bimetallic catalyst nanoparticles by rod-to-tube type spark discharge generator. J. Aerosol Sci. 2018, 123, 208–218. [Google Scholar] [CrossRef]
- Stein, M.; Kruis, F.E. Scaling-up metal nanoparticle production by transferred arc discharge. Adv. Powder Technol. 2018, 29, 3138–3144. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Shegai, T.; Chen, S.; Miljković, V.D.; Zengin, G.; Johansson, P.; Käll, M. A Bimetallic Nanoantenna for Directional Colour Routing. Nat. Commun. 2011, 2, 481. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [Green Version]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Evans, D.E.; Harrison, R.M.; Ayres, J.G. The Generation and Characterization of Metallic and Mixed Element Aerosols for Human Challenge Studies. Aerosol Sci. Technol. 2003, 37, 975–987. [Google Scholar] [CrossRef]
- Byeon, J.H.; Park, J.H.; Hwang, J. Spark Generation of Monometallic and Bimetallic Aerosol Nanoparticles. Aerosol Sci. 2008, 39, 888–896. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Xu, Q.; van der Pers, N.M.; Lafont, U.; Schmidt-Ott, A. Synthesis of Mixed Metallic Nanoparticles by Spark Discharge. J. Nanopart. Res. 2009, 11, 1209. [Google Scholar] [CrossRef]
- Song, A.J.; Ma, M.Z.; Zhang, W.G.; Zong, H.T.; Liang, S.X.; Hao, Q.H.; Zhou, R.Z.; Jing, Q.; Liu, R.P. Preparation and Growth of Ni–Cu Alloy Nanoparticles Prepared by Arc Plasma Evaporation. Mater. Lett. 2010, 64, 1229–1231. [Google Scholar] [CrossRef]
- Feng, J.; Ramlawi, N.; Biskos, G.; Schmidt-Ott, A. Internally Mixed Nanoparticles from Oscillatory Spark Ablation between Electrodes of Different Materials. Aerosol Sci. Technol. 2018, 52, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lee, C.; Schmidt, L.D. Shape and Orientation of Supported Pt Particles. Surf. Sci. 1985, 163, 181–197. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Peng, H.-C. Shape-Controlled Synthesis of Colloidal Metal Nanocrystals: Thermodynamic versus Kinetic Products. J. Am. Chem. Soc. 2015, 137, 7947–7966. [Google Scholar] [CrossRef]
- Craig, S.; Harding, G.L. Effects of argon pressure and substrate temperature on the structure and properties of sputtered copper films. J. Vac. Sci. Technol. 1981, 19, 205–215. [Google Scholar] [CrossRef]
- West, G.T.; Kelly, P.J. Influence of inert gas species on the growth of silver and molybdenum films via a magnetron discharge. Surf. Coat. Technol. 2011, 206, 1648–1652. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Yang, H.; Suh, J.; Kim, T.; Han, B.; Kim, S.; Kim, D.S.; Pikhitsa, P.V.; Choi, M. Parallel Patterning of Nanoparticles via Electrodynamic Focusing of Charged Aerosols. Nat. Nanotechnol. 2006, 1, 117–121. [Google Scholar] [CrossRef]
- Ha, K.; Choi, H.; Jung, K.; Han, K.; Lee, J.-K.; Ahn, K.; Choi, M. Large-Area Assembly of Three-Dimensional Nanoparticle Structures via Ion Assisted Aerosol Lithography with a Multi-Pin Spark Discharge Generator. Nanotechnology 2014, 25, 225302. [Google Scholar] [CrossRef]
- Sung, H.; Choi, M. Assembly of Nanoparticles: Towards Multiscale Three-Dimensional Architecturing. KONA Powder Part. J. 2013, 30, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Prost, W.; Kruis, F.E.; Otten, F.; Nielsch, K.; Rellinghaus, B.; Auer, U.; Peled, A.; Wassermann, E.F.; Fissan, H.; Tegude, F.J. Monodisperse Aerosol Particle Deposition: Prospects for Nanoelectronics. Microelectron. Eng. 1998, 41–42, 535–538. [Google Scholar] [CrossRef]
- Krinke, T.J.; Deppert, K.; Magnusson, M.H.; Fissan, H. Nanostructured Deposition of Nanoparticles from the Gas Phase. Part. Part. Syst. Charact. 2002, 19, 321–326. [Google Scholar] [CrossRef]
- Jacobs, H.O.; Campbell, S.A.; Steward, M.G. Approaching Nanoxerography: The Use of Electrostatic Forces to Position Nanoparticles with 100 nm Scale Resolution. Adv. Mater. 2002, 14, 1553–1557. [Google Scholar] [CrossRef]
- Wang, X.; Kruis, F.E.; McMurry, P.H. Aerodynamic Focusing of Nanoparticles: I. Guidelines for Designing Aerodynamic Lenses for Nanoparticles. Aerosol Sci. Technol. 2005, 39, 611–623. [Google Scholar] [CrossRef]
- Cole, J.J.; Lin, E.-C.; Barry, C.R.; Jacobs, H.O. Continuous Nanoparticle Generation and Assembly by Atmospheric Pressure Arc Discharge. Appl. Phys. Lett. 2009, 95, 113101. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz de Zárate, D.; García-Meca, C.; Pinilla-Cienfuegos, E.; Ayúcar, J.A.; Griol, A.; Bellières, L.; Hontañón, E.; Kruis, F.E.; Martí, J. Green and Sustainable Manufacture of Ultrapure Engineered Nanomaterials. Nanomaterials 2020, 10, 466. https://doi.org/10.3390/nano10030466
Ortiz de Zárate D, García-Meca C, Pinilla-Cienfuegos E, Ayúcar JA, Griol A, Bellières L, Hontañón E, Kruis FE, Martí J. Green and Sustainable Manufacture of Ultrapure Engineered Nanomaterials. Nanomaterials. 2020; 10(3):466. https://doi.org/10.3390/nano10030466
Chicago/Turabian StyleOrtiz de Zárate, David, Carlos García-Meca, Elena Pinilla-Cienfuegos, José A. Ayúcar, Amadeu Griol, Laurent Bellières, Esther Hontañón, Frank E. Kruis, and Javier Martí. 2020. "Green and Sustainable Manufacture of Ultrapure Engineered Nanomaterials" Nanomaterials 10, no. 3: 466. https://doi.org/10.3390/nano10030466
APA StyleOrtiz de Zárate, D., García-Meca, C., Pinilla-Cienfuegos, E., Ayúcar, J. A., Griol, A., Bellières, L., Hontañón, E., Kruis, F. E., & Martí, J. (2020). Green and Sustainable Manufacture of Ultrapure Engineered Nanomaterials. Nanomaterials, 10(3), 466. https://doi.org/10.3390/nano10030466