A Study of a PID Controller Used in a Micro-Electrical Discharge Machining System to Prepare TiO2 Nanocolloids
Abstract
:1. Introduction
2. Materials and Methods
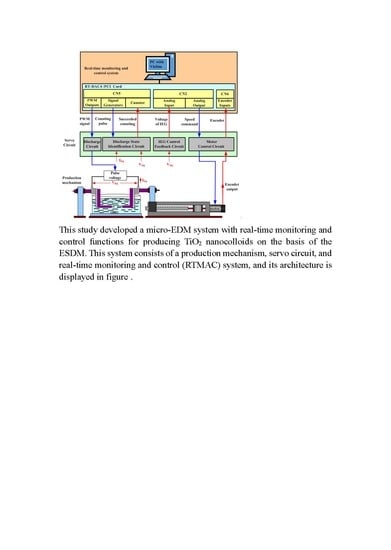
2.1. The Micro-EDM System
- IEG control feedback circuit: the signal required for closed-loop control of the IEG is captured.
- Discharge state identification circuit: this circuit is used to determine the discharge state and provide the signal required to calculate the discharge success rate (DSR).
2.2. Proportional–Integral–Derivative (PID) Controller of the IEG and the ITAE Performance Criterion
2.2.1. Design of the PID Controller by Using the Root Locus Method
2.2.2. Design of the PID Controller by Using the Z–N Method
2.2.3. Evaluation of the ITAE Performance Criterion for the PID Controller
2.3. RTMAC System in the Micro-EDM System
2.4. The Settings of the Apparatus
3. Results and Discussion
3.1. Evaluation of the PID Controller for the Production of TiO2 Nanocolloids Designed Using the Root Locus Method
3.2. Evaluation of the PID Controller for the Production of TiO2 Nanocolloids Designed Using the Z–N Method
4. Conclusions
- The micro-EDM system successfully produced TiO2 nanocolloids with excellent suspension stability under room temperature and atmospheric pressure, and colloids contained no elements other than Ti and O. Their application in environmental pollution prevention would help prevent secondary pollution to the environment.
- Compared with industrial-grade electrical discharge machines, the micro-EDM system has the advantages of small size and low cost. These factors can enhance the advantages of the ESDM in the production of TiO2 NPs.
- The parameters of the PID controller designed using the Z–N method (Kp = 0.3894, Ki = 2.13956 and Kd = 0.017718) were obtained through the online adjustment method. This method considers the electrode material and characteristics of the dielectric fluid. Therefore, the controller‘s efficiency at controlling the IEG was superior to that exerted by the PID controller designed using the root locus method.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gélis, C.; Girard, S.; Mavon, A.; Delverdier, M.; Paillous, N.; Vicendo, P. Assessment of the skin photoprotective capacities of an organo-mineral broad-spectrum sunblock on two ex vivo skin models. Photodermatol. Photoimmunol. Photomed. 2003, 19, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, S.; Pan, N. Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar] [CrossRef]
- Huang, P.; Xu, K.W.; Han, Y. Preparation and apatite layer formation of plasma electrolytic oxidation film on titanium for biomedical application. Mater. Lett. 2005, 59, 185–189. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- San, N.; Hatipoğlu, A.; Koçtürk, G.; Çınar, Z. Photocatalytic degradation of 4-nitrophenol in aqueous TiO2 suspensions: Theoretical prediction of the intermediates. J. Photochem. Photobiol. A Chem. 2002, 146, 189–197. [Google Scholar]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.S.; Lee, J.C.; Lee, H.; Eom, S.Y.; Yoo, E.A. Synthesis of highly concentrated TiO2 nanocolloids and coating on boron nitride powders. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 175–178. [Google Scholar] [CrossRef]
- Karimipour, M. Comparison of Acidic and Polymeric Agents in Synthesis of TiO2 Nanoparticles via a Modified Sol-Gel Method. World J. Nano Sci. Eng. 2013, 2013, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.K.; Chen, J.J.; Zhang, X.; Huang, Y.X.; Li, W.W.; Yu, H.Q. Self-induced synthesis of phase-junction TiO 2 with a tailored rutile to anatase ratio below phase transition temperature. Sci. Rep. 2016, 6, 20491. [Google Scholar] [CrossRef] [Green Version]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [PubMed] [Green Version]
- Hockenberry, T.O. The Role of the Dielectric Fluid in Electrical Discharge Machining. SAE Tech. Pap. 1968. [Google Scholar] [CrossRef]
- Tseng, K.H.; Chang, C.Y.; Chung, M.Y.; Cheng, T.S. Fabricating TiO2 nanocolloids by electric spark discharge method at normal temperature and pressure. Nanotechnology 2017, 28, 465701. [Google Scholar]
- Minhat, A.E.B.; Hj, N.H.B.; Yahya, A.B.; Andromeda, T.; Nugroho, K. Model of pulsed electrical discharge machining (EDM) using RL circuit. Int. J. Power Electron. Drive Syst. 2014, 2, 252. [Google Scholar] [CrossRef]
- Gostimirovic, M.; Kovac, P.; Sekulic, M.; Skoric, B. Influence of discharge energy on machining characteristics in EDM. J. Mech. Sci. Technol. 2012, 26, 173–179. [Google Scholar] [CrossRef]
- De Bruyn, H.E.; Pekelharing, A.J. Has the «Delay Time» Influence on the EDM-Process? Cirp Ann. 1982, 31, 103–106. [Google Scholar]
- Hontañón, E.; Palomares, J.M.; Stein, M.; Guo, X.; Engeln, R.; Nirschl, H.; Kruis, F.E. The transition from spark to arc discharge and its implications with respect to nanoparticle production. J. Nanoparticle Res. 2013, 15, 1–19. [Google Scholar]
- Tien, D.-C.; Liao, C.-Y.; Huang, J.-C.; Tseng, K.-H.; Lung, J.-K.; Tsung, T.-T.; Kao, W.-S.; Tsai, T.-H.; Cheng, T.-W.; Yu, B.-S.; et al. Novel Technique for Preparing a Nano-Silver Water Suspension by the Arc-Discharge Method. Rev. Adv. Mater. Sci. 2008, 18, 750–756. [Google Scholar]
- Chang, H.; Tsung, T.T.; Yang, Y.C.; Chen, L.C.; Lin, H.M.; Lin, C.K.; Jwo, C.S. Nanoparticle suspension preparation using the arc spray nanoparticle synthesis system combined with ultrasonic vibration and rotating electrode. Int. J. Adv. Manuf. Technol. 2005, 26, 552–558. [Google Scholar] [CrossRef]
- Gołdasz, J.; Sapinski, B.; Jastrzębski, Ł. Assessment of the magnetic hysteretic behaviour of MR dampers through sensorless measurements. Shock Vib. 2018, 2018, 3740208. [Google Scholar] [CrossRef]
- Ustun, O.; Tuncay, R.N. Design, analysis, and control of a novel linear actuator. IEEE Trans. Ind. Appl. 2006, 42, 1007–1013. [Google Scholar]
- Toma, E.; Simion, C. The improvement of edm pulse generator performances by use of the news silicon-carbide mos transistors. Nonconv. Technol. Rev. /Rev. De Tehnol. Neconv. 2016, 20. [Google Scholar]
- Erawan, M.A.; Yahya, A.; Nor Hisham, K.; Samosir, A.S.; Rahim, M.A.A.; Trias, A.; Kartiko, J.P.N. Model of pulse power generator in electrical discharge machining (EDM) system. Appl. Mech. Mater. 2014, 554, 613–617. [Google Scholar]
- Bhagwatkar, S.R.; Dhande, A.P. A review on automatic closed loop speed control of a DC motor. Int. J. Adv. Res. Comput. Commun. Eng. 2015, 4, 137–139. [Google Scholar] [CrossRef]
- Hashim, N.L.S.; Yahya, A.; Daud, M.R.; Syahrullail, S.; Baharom, A.; Khamis, N.H.; Mahmud, N. Review on an electrical discharge machining servomechanism system. Sci. Iran. Trans. B Mech. Eng. 2015, 22, 1813. [Google Scholar]
- Mohamed, T.L.T.; Mohamed, R.H.A.; Mohamed, Z. Development of auto tuning PID controller using graphical user interface (GUI). Int. J. Inf. Electron. Eng. 2013, 3, 382–385. [Google Scholar]
- Andromeda, T.; Yahya, A.; Samion, S.; Baharom, A.; Hashim, N.L. Differential evolution for optimization of PID gain in Electrical Discharge Machining control system. Trans. Can. Soc. Mech. Eng. 2013, 37.3, 293–301. [Google Scholar] [CrossRef]
- Kalaiselvan, E.; Tagore, J.D. A comparative novel method of tuning of controller for temperature process. Int. J. Adv. Res. Electr. Electron. Instrum. Eng. 2013, 2. [Google Scholar]
- Sudha, G.; Deepa, S.N. Optimization for pid control parameters on pitch control of aircraft dynamics based on tuning methods. Appl. Math. Inf. Sci. 2016, 10, 343–350. [Google Scholar]
- Babu, R.; Swarnalath, R. Comparison of Different Tuning Methods for pH Neutralization in Textile Industry. J. Appl. Sci. 2017, 17, 142–147. [Google Scholar] [CrossRef]
- Basilio, J.C.; Matos, S.R. Design of PI and PID controllers with transient performance specification. IEEE Trans. Educ. 2002, 364–370. [Google Scholar] [CrossRef]
- Sahib, M.A.; Ahmed, B.S. A new multiobjective performance criterion used in PID tuning optimization algorithms. J. Adv. Res. 2016, 7, 125–134. [Google Scholar] [PubMed] [Green Version]
- Hodzic, M. Grey Predictor Reference Model for Assisting Particle Swarm Optimization. Southeast Eur. J. Soft Comput. 2014, 3, 39–44. [Google Scholar] [CrossRef]
- Hashim, N.L.S.; Yahya, A.; Andromeda, T.; Kadir, M.R.R.A.; Mahmud, N.; Samion, S. Simulation of PSO-PI Co ontroller of DC Motor in Micro—EDM System for Biomedical Application. Procedia Eng. 2012, 41, 805–811. [Google Scholar]
- Martins, F.G. Tuning PID controllers using the ITAE criterion. Int. J. Eng. Educ. 2005, 21, 867–873. [Google Scholar]
- Omer Shuaib, A.; Mohamed Ahmed, M. Robust PID control system design using ITAE performance index (DC motor model). Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15060–15067. [Google Scholar]
- Awouda, E.A.; Mamat, R.B. New PID tuning rule using ITAE criteria. Int. J. Eng. (IJE) 2010, 3, 597–608. [Google Scholar]














| 0.6 | /2 | /8 |
| Mode Type | PID Controller | Ra (Ω) | La (mH) | |||
|---|---|---|---|---|---|---|
| Mode 1 | PID-M1 | 3.45 | 1.84 | |||
| Mode 2 | PID-M2 | 3.45 | 2.23 | |||
| Mode 3 | PID-M3 | 3.45 | 3.89 | |||
| Mode 4 | PID-M4 | 7.25 | 1.84 | |||
| Mode 5 | PID-M5 | 7.25 | 2.23 | |||
| Mode 6 | PID-M6 | 7.25 | 3.89 | |||
| Mode 7 | PID-M7 | 9.15 | 1.84 | |||
| Mode 8 | PID-M8 | 9.15 | 2.23 | |||
| Mode 9 | PID-M9 | 9.15 | 3.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, K.-H.; Lin, Y.-S.; Chang, C.-Y.; Chung, M.-Y. A Study of a PID Controller Used in a Micro-Electrical Discharge Machining System to Prepare TiO2 Nanocolloids. Nanomaterials 2020, 10, 1044. https://doi.org/10.3390/nano10061044
Tseng K-H, Lin Y-S, Chang C-Y, Chung M-Y. A Study of a PID Controller Used in a Micro-Electrical Discharge Machining System to Prepare TiO2 Nanocolloids. Nanomaterials. 2020; 10(6):1044. https://doi.org/10.3390/nano10061044
Chicago/Turabian StyleTseng, Kuo-Hsiung, Yur-Shan Lin, Chaur-Yang Chang, and Meng-Yun Chung. 2020. "A Study of a PID Controller Used in a Micro-Electrical Discharge Machining System to Prepare TiO2 Nanocolloids" Nanomaterials 10, no. 6: 1044. https://doi.org/10.3390/nano10061044
APA StyleTseng, K. -H., Lin, Y. -S., Chang, C. -Y., & Chung, M. -Y. (2020). A Study of a PID Controller Used in a Micro-Electrical Discharge Machining System to Prepare TiO2 Nanocolloids. Nanomaterials, 10(6), 1044. https://doi.org/10.3390/nano10061044