Comparison of the Corrosion Behavior of Brass in TiO2 and Al2O3 Nanofluids
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. The Experimental Medium
2.3. Electrochemical Measurements
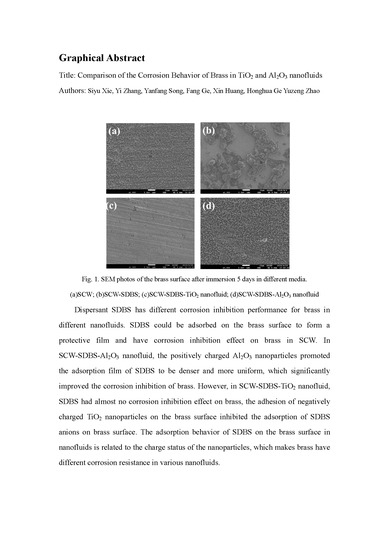
2.4. Characterization of the Metal Surface
3. Results and Discussion
3.1. Stability Analysis of TiO2 and Al2O3 Nanofluids
3.2. EIS Analysis
3.3. Potentiodynamic Polarization Analysis
3.4. Corrosion Products Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sidik, N.A.C.; Mohammed, H.A.; Alawi, O.A.; Samion, S. A review on preparation methods and challenges of nanofluids. Int. Commun. Heat Mass Transf. 2014, 54, 115–125. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.-K.; Lee, C.H.; Jung, Y.; Cheong, S.; Lee, C.; Ku, B.; Jang, S. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Kumar, S.; Dinesha, P.; Gaggad, A.; Mehrotra, K. Performance investigation of an automotive car radiator operated with nanofluid based coolant. Heat Transf. Res. 2018, 49, 1527–1543. [Google Scholar] [CrossRef]
- Zamzamian, A.; KeyanpourRad, M.; KianiNeyestani, M.; Jamal-Abad, M.T. An experimental study on the effect of Cu-synthesized/EG nanofluid on the efficiency of flat-plate solar collectors. Renew. Energy 2014, 71, 658–664. [Google Scholar] [CrossRef]
- Bi, S.; Guo, K.; Liu, Z.; Wu, J. Performance of a domestic refrigerator using TiO2-R600a nano-refrigerant as working fluid. Energy Convers. Manag. 2011, 52, 733–737. [Google Scholar] [CrossRef]
- Raja, M.; Vijayan, R.; Dineshkumar, P.; Venkatesan, M. Review on nanofluids characterization, heat transfer characteristics and applications. Renew. Sustain. Energy Rev. 2016, 64, 163–173. [Google Scholar] [CrossRef]
- Rashidi, A.; Amrollahi, A.; Lotfi, R.; Javaheryzadeh, H.; Rahimi, H.; Rahimi, A.R.; Jorsaraei, A. An investigation of electrochemical behavior of nanofluids containing MWCNT on the corrosion rate of carbon steel. Mater. Res. Bull. 2013, 48, 4438–4443. [Google Scholar] [CrossRef]
- Celata, G.P.; D’Annibale, F.; Mariani, A.; Sau, S.; Serra, E.; Bubbico, R.; Menale, C.; Poth, H. Experimental results of nanofluids flow effects on metal surfaces. Chem. Eng. Res. Des. 2014, 92, 1616–1628. [Google Scholar] [CrossRef]
- Fotowat, S.; Askar, S.; Ismail, M.; Fartaj, A. A study on corrosion effects of a water based nanofluid for enhanced thermal energy applications. Sustain. Energy Technol. Assessments 2017, 24, 39–44. [Google Scholar] [CrossRef]
- Bubbico, R.; Celata, G.P.; D’Annibale, F.; Mazzarotta, B.; Menale, C. Experimental analysis of corrosion and erosion phenomena on metal surfaces by nanofluids. Chem. Eng. Res. Des. 2015, 104, 605–614. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Grosu, Y.; González-Fernández, L.; Zaki, A.; Igartua, J.M.; Faik, A. Corrosion aspects of molten nitrate salt-based nanofluids for thermal energy storage applications. Sol. Energy 2019, 189, 219–227. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Hu, H. Effect of surfactant additives on nucleate pool boiling heat transfer of refrigerant-based nanofluid. Exp. Therm. Fluid Sci. 2011, 35, 960–970. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Du, K.; Niu, X.; Yang, L.; Zhang, Y. An experimental and theoretical study of the influence of surfactant on the preparation and stability of ammonia-water nanofluids. Int. J. Refrig. 2011, 34, 1741–1748. [Google Scholar] [CrossRef]
- Yang, L.; Du, K.; Zhang, X.-S. Influence factors on thermal conductivity of ammonia-water nanofluids. J. Central South Univ. 2012, 19, 1622–1628. [Google Scholar] [CrossRef]
- Said, Z.; Sabiha, M.; Saidur, R.; Hepbasli, A.; Rahim, N.; Mekhilef, S.; Ward, T. Performance enhancement of a Flat Plate Solar collector using Titanium dioxide nanofluid and Polyethylene Glycol dispersant. J. Clean. Prod. 2015, 92, 343–353. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; El-Lateef, H.M.A. Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2-3.5%NaCl: DFT, Monte Carlo simulations and experimental approaches. J. Clean. Prod. 2020, 250, 119510. [Google Scholar] [CrossRef]
- Sha, J.-Y.; Ge, H.; Wan, C.; Wang, L.-T.; Xie, S.-Y.; Meng, X.-J.; Zhao, Y.-Z. Corrosion inhibition behaviour of sodium dodecyl benzene sulphonate for brass in an Al2O3 nanofluid and simulated cooling water. Corros. Sci. 2019, 148, 123–133. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.-S.; Li, S.; Eastman, J. Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Ismay, M.J.; Doroodchi, E.; Moghtaderi, B. Effects of colloidal properties on sensible heat transfer in water-based titania nanofluids. Chem. Eng. Res. Des. 2013, 91, 426–436. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Siahmargoi, M.; Asadi, T.; Andarati, M.G. The effect of surfactant and sonication time on the stability and thermal conductivity of water-based nanofluid containing Mg(OH)2 nanoparticles: An experimental investigation. Int. J. Heat Mass Transf. 2017, 108, 191–198. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Wang, X.-J.; Zhu, D.-S.; Yang, S. Investigation of pH and SDBS on enhancement of thermal conductivity in nanofluids. Chem. Phys. Lett. 2009, 470, 107–111. [Google Scholar] [CrossRef]
- Yang, L.; Du, K.; Zhang, X.S.; Cheng, B. Preparation and stability of Al2O3 nano-particle suspension of ammonia–water solution. Appl. Therm. Eng. 2011, 31, 3643–3647. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.; Mehrali, M.; Zubir, M.N.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of chloroform and other organic molecules in aqueous TiO2 suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Kao, J.-Y.; Wu, T.-M.; Cheng, W.T. Dispersion of Titanium Oxide Nanoparticles in Aqueous Solution with Anionic Stabilizer via Ultrasonic Wave. J. Nanopart. 2016, 2016, 6539581. [Google Scholar] [CrossRef]
- Fan, H.; Li, S.; Zhao, Z.; Wang, H.; Shi, Z.; Zhang, L. Inhibition of brass corrosion in sodium chloride solutions by self-assembled silane films. Corros. Sci. 2011, 53, 4273–4281. [Google Scholar] [CrossRef]
- Emregül, K.C.; Atakol, O. Corrosion inhibition of mild steel with Schiff base compounds in 1 M HCl. Mater. Chem. Phys. 2003, 82, 188–193. [Google Scholar] [CrossRef]
- Ma, X.; Xu, L.; Wang, W.; Lin, Z.; Li, X. Synthesis and characterisation of composite nanoparticles of mesoporous silica loaded with inhibitor for corrosion protection of Cu-Zn alloy. Corros. Sci. 2017, 120, 139–147. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, B.; Liang, Y.; Song, S.; Liu, C. Corrosion control of copper in 3.5wt.% NaCl Solution by Domperidone: Experimental and Theoretical Study. Corros. Sci. 2014, 85, 77–86. [Google Scholar] [CrossRef]
- Wu, K.; Ge, H.-H.; Wang, F.; Zhou, H.-W. Corrosion Behavior of Brass In TiO2 Nanofluids. In Proceedings of the Materials Science and Engineering Conference Series 2017, Barcelona, Spain, 14 July–16 August 2017. [Google Scholar] [CrossRef]
- Yuan, Q.; Ge, H.-H.; Sha, J.-Y.; Wang, L.-T.; Wan, C.; Wang, F.; Wu, K.; Meng, X.-J.; Zhao, Y.-Z. Influence of Al2O3 nanoparticles on the corrosion behavior of brass in simulated cooling water. J. Alloy. Compd. 2018, 764, 512–522. [Google Scholar] [CrossRef]








| Elements | Cu | Sn | Fe | Pb | As | Bi | P | Zn |
|---|---|---|---|---|---|---|---|---|
| Contents | 69.9 | 0.90 | 0.10 | 0.05 | 0.04 | 0.002 | 0.01 | the rest |
| CSDBS (mg/L) | 0 | 500 |
|---|---|---|
| SCW-SDBS-TiO2 nanofluid | −19.8 | −46.4 |
| SCW-SDBS-Al2O3 nanofluid | 3.28 | −40.9 |
| Test Media | Rs | Rf | Qf | n1 | Rct | Qdl | n2 |
|---|---|---|---|---|---|---|---|
| Ω·cm2 | kΩ·cm2 | Yf(μS·sncm−2) | kΩ·cm2 | Ydl(μS·sncm−2) | |||
| SCW | 162.1 | 10.59 | 28.71 | 0.80 | 44.70 | 53.57 | 0.58 |
| SCW-SDBS | 173.8 | 21.01 | 23.44 | 0.74 | 163.5 | 32.25 | 0.85 |
| SCW-SDBS-TiO2 | 131.7 | 13.58 | 26.57 | 0.75 | 62.61 | 48.82 | 0.65 |
| SCW-SDBS-Al2O3 | 150.0 | 27.27 | 18.94 | 0.87 | 266.1 | 27.17 | 0.75 |
| Test Media | Ecorr | ba | bc | jcorr |
|---|---|---|---|---|
| (mV) | (mV dec−1) | (mV dec−1) | (μA·cm−2) | |
| SCW | −87 | 103 | 250 | 0.388 |
| SCW-SDBS | −154 | 188 | 174 | 0.161 |
| SCW-SDBS-TiO2 | −125 | 95 | 157 | 0.302 |
| SCW-SDBS-Al2O3 | −171 | 192 | 197 | 0.105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Zhang, Y.; Song, Y.; Ge, F.; Huang, X.; Ge, H.; Zhao, Y. Comparison of the Corrosion Behavior of Brass in TiO2 and Al2O3 Nanofluids. Nanomaterials 2020, 10, 1046. https://doi.org/10.3390/nano10061046
Xie S, Zhang Y, Song Y, Ge F, Huang X, Ge H, Zhao Y. Comparison of the Corrosion Behavior of Brass in TiO2 and Al2O3 Nanofluids. Nanomaterials. 2020; 10(6):1046. https://doi.org/10.3390/nano10061046
Chicago/Turabian StyleXie, Siyu, Yi Zhang, Yanfang Song, Fang Ge, Xin Huang, Honghua Ge, and Yuzeng Zhao. 2020. "Comparison of the Corrosion Behavior of Brass in TiO2 and Al2O3 Nanofluids" Nanomaterials 10, no. 6: 1046. https://doi.org/10.3390/nano10061046
APA StyleXie, S., Zhang, Y., Song, Y., Ge, F., Huang, X., Ge, H., & Zhao, Y. (2020). Comparison of the Corrosion Behavior of Brass in TiO2 and Al2O3 Nanofluids. Nanomaterials, 10(6), 1046. https://doi.org/10.3390/nano10061046