Effect of Chemical Solvents on the Wetting Behavior Over Time of Femtosecond Laser Structured Ti6Al4V Surfaces
Abstract
:1. Introduction
2. Materials and Methods
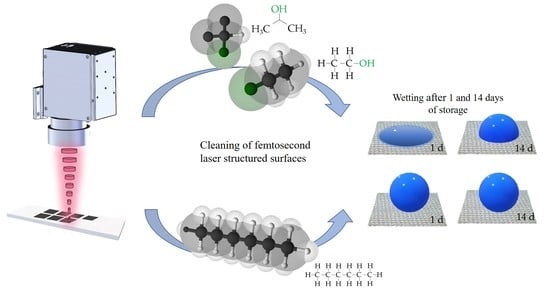
2.1. Materials and Cleaning Procedure
2.2. Laser Treatment
2.3. Storage Conditions
2.4. Surface Wettability
2.5. Surface Characterization
2.6. Surface Chemical Analysis via XPS
3. Results and Discussion
3.1. Surface Topography
3.2. Contact Angle Measurements
3.3. Chemical Composition of the Surfaces and Its Effects on the Wetting State
4. Conclusions
- The wetting behavior of laser structured surfaces clearly depends on the used cleaning solvent.
- Alcohols possess OH− groups that affect the composition of structured surfaces and evoke a temporary hydrophilic behavior after laser treatment.
- The cleaning in hexane provides the highest SCAs after laser treatment within 14 days after laser treatment due to the initial adsorption of long-chained hydrocarbons.
- The cleaning of the surfaces after laser irradiation plays a major role in the formation of the SCA. Thus, SCAs should only be compared to each other where identical post-processing methods have been applied.
- The effect of cleaning with different solvents and adsorption of carbon on the formation of the SCA should be considered for all correlations between surface and secondary effects.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LT | laser treatment |
| SCA | static contact angle |
| US | ultrasonic bath |
Appendix A
| Structured | Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cleaning | Pure Water | Ethanol | Acetone | Isopropanol | Hexane | No Solvent | No Solvent | ||||||||
| Time of storage (days) | 1 | 14 | 1 | 14 | 1 | 14 | 1 | 14 | 1 | 14 | 1 | 14 | 1 | 14 | |
| Al 2p | Al3+ (at.%) | 8.05 | 8.21 | 8.38 | 7.54 | 8.05 | 7.77 | 7.95 | 7.79 | 6.81 | 7.06 | 8.83 | 8.65 | 3.15 | 3.08 |
| Al0 (at.%) | 0.61 | 0.69 | 0.66 | 0.42 | 0.53 | 0.5 | 0.61 | 0.4 | 0.59 | 0.5 | 0.65 | 0.59 | 0.69 | 0.66 | |
| Al Total (at.%) | 8.66 | 8.9 | 9.04 | 7.96 | 8.58 | 8.27 | 8.56 | 8.19 | 7.4 | 7.56 | 9.48 | 9.24 | 3.84 | 3.74 | |
| C 1s | C–C/C– (at.%) | 17.36 | 19.32 | 17.25 | 23.26 | 19.19 | 21.11 | 21.61 | 22.1 | 28.19 | 23.94 | 14.56 | 16.66 | 12.1 | 13.95 |
| –C–OH (at.%) | 4.72 | 5.27 | 4.24 | 5.14 | 5.96 | 5.66 | 4.34 | 5.3 | 4.29 | 4.42 | 3.9 | 4.81 | 3.56 | 3.75 | |
| –C=O (at.%) | 0.88 | 1.45 | 1.14 | 1.5 | 1.13 | 1.47 | 1.3 | 1.79 | 2.25 | 2.82 | 0.69 | 1.23 | 1.83 | 1.75 | |
| O=CO– (at.%) | 2.46 | 3.56 | 2.25 | 3.09 | 2.1 | 2.84 | 2.26 | 2.81 | 2.91 | 4.02 | 2.01 | 2.83 | 5.24 | 5.1 | |
| Pi–Pi (at.%) | 0.19 | 0.05 | 0.01 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C Total (at.%) | 25.61 | 29.65 | 24.89 | 32.99 | 28.38 | 31.13 | 29.51 | 32.00 | 37.64 | 35.20 | 21.16 | 25.53 | 22.73 | 24.55 | |
| O 1s | TiO2 (at.%) | 31.2 | 27.83 | 31.75 | 27.5 | 29.22 | 27.38 | 30.2 | 27.25 | 25.73 | 25.74 | 33.07 | 30.8 | 34.21 | 32.35 |
| Al2O3/C=O (at.%) | 9.67 | 10.34 | 10.04 | 9.09 | 10.06 | 9.02 | 8.98 | 8.79 | 9.41 | 9.35 | 10.27 | 10.45 | 9.14 | 9.02 | |
| O–C/OH– (at.%) | 8.43 | 9.26 | 8.03 | 8.62 | 8.19 | 8.19 | 7.71 | 7.83 | 6.9 | 9.42 | 8.63 | 8.59 | 11.35 | 11.36 | |
| C–O–C/C–OH (at.%) | 2.25 | 2.13 | 2.19 | 2,25 | 2.1 | 2.12 | 1.94 | 2.19 | 1.43 | 2.33 | 2.26 | 1.98 | 2.82 | 3.17 | |
| O Total (at.%) | 51.55 | 49.56 | 52.01 | 47.46 | 49.57 | 46.71 | 48.83 | 46.06 | 43.47 | 46.84 | 54.23 | 51.82 | 57.52 | 55.9 | |
| Ti 2p | Ti4+ (at.%) | 13.4 | 11.42 | 13.24 | 11.04 | 12.53 | 13.13 | 12.35 | 12.98 | 10.61 | 9.71 | 14.18 | 12.78 | 15.19 | 15.07 |
| Ti0 (at.%) | 0.77 | 0.49 | 0.82 | 0.53 | 0.93 | 0.77 | 0.74 | 0.78 | 0.87 | 0.67 | 0.95 | 0.63 | 0.72 | 0.73 | |
| Ti Total (at.%) | 14.17 | 11.91 | 14.06 | 11.57 | 13.46 | 13.9 | 13.09 | 13.76 | 11.48 | 10.38 | 15.13 | 13.41 | 15.91 | 15.8 | |
Appendix B







References
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Shin, S.; Seo, J.; Han, H.; Kang, S.; Kim, H.; Lee, T. Bio-inspired extreme wetting surfaces for biomedical applications. Materials 2016, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Parkin, I.P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Ice friction: The effects of surface roughness, structure, and hydrophobicity. J. Appl. Phys. 2009, 106, 24303. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Synergistic Effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mater. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.A.; Coppage-Gross, J.; Rangan, K.K.; Onyilo, V.C.; Sudarshan, T.S.; Singh, S.R.; Pillai, S.R. Multifunctionally modified superhydrophobic aluminum and fabric surfaces with reduced gram-negative and gram-positive bacterial attachment: A possible approach for self-cleaning aircraft and crew cabin surfaces. Mater. Manuf. Process. 2016, 31, 1156–1161. [Google Scholar] [CrossRef]
- Linklater, D.P.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P.; Crawford, R. Mechanical inactivation of Staphylococcus aureus and Pseudomonas aeruginosa by titanium substrata with hierarchical surface structures. Materialia 2019, 5, 100197. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N.; Rubanov, S.; Hanssen, E.; Wandiyanto, J.; Truong, V.K.; et al. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Multifunctional surfaces produced by femtosecond laser pulses. J. Appl. Phys. 2015, 117, 33103. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.Y.; Vorobyev, A.Y.; Guo, C. Enhanced efficiency of solar-driven thermoelectric generator with femtosecond laser-textured metals. Opt. Express 2011, 19, A824–A829. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.Y.; Guo, C. Femtosecond laser structuring of titanium implants. Appl. Surf. Sci. 2007, 253, 7272–7280. [Google Scholar] [CrossRef]
- Schnell, G.; Staehlke, S.; Duenow, U.; Nebe, J.B.; Seitz, H. Femtosecond laser nano/micro textured Ti6Al4V surfaces-effect on wetting and MG-63 cell adhesion. Materials 2019, 12, 2210. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.; Ausset, S.; Vilar, R. Surface micro/nanostructuring of titanium under stationary and non-stationary femtosecond laser irradiation. Appl. Surf. Sci. 2009, 255, 7556–7560. [Google Scholar] [CrossRef]
- Moradi, S.; Kamal, S.; Englezos, P.; Hatzikiriakos, S.G. Femtosecond laser irradiation of metallic surfaces: Effects of laser parameters on superhydrophobicity. Nanotechnology 2013, 24, 415302. [Google Scholar] [CrossRef]
- Schnell, G.; Duenow, U.; Seitz, H. Effect of laser pulse overlap and scanning line overlap on femtosecond laser-structured Ti6Al4V surfaces. Materials 2020, 13, 969. [Google Scholar] [CrossRef] [Green Version]
- Schnell, G.; Jagow, C.; Springer, A.; Frank, M.; Seitz, H. Time-dependent anisotropic wetting behavior of deterministic structures of different strut widths on Ti6Al4V. Metals 2019, 9, 938. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Hu, H.; Li, Z.; Shen, Y.; Xu, Y.; Zhang, G.; Zeng, X.; Deng, J.; Zhao, S.; Ren, T.; et al. Enhanced osseointegration of titanium alloy implants with laser microgrooved surfaces and graphene oxide coating. ACS Appl. Mater. Interfaces 2019, 11, 39470–39483. [Google Scholar] [CrossRef] [PubMed]
- Rukosuyev, M.V.; Lee, J.; Cho, S.J.; Lim, G.; Jun, M.B.G. One-step fabrication of superhydrophobic hierarchical structures by femtosecond laser ablation. Appl. Surf. Sci. 2014, 313, 411–417. [Google Scholar] [CrossRef]
- Schlie, S.; Fadeeva, E.; Koch, J.; Ngezahayo, A.; Chichkov, B.N. Femtosecond laser fabricated spike structures for selective control of cellular behavior. J. Biomater. Appl. 2010, 25, 217–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadeeva, E.; Schlie, S.; Koch, J.; Ngezahayo, A.; Chichkov, B.N. The hydrophobic properties of femtosecond laser fabricated spike structures and their effects on cell proliferation. Phys. Status Solidi (a) 2009, 206, 1348–1351. [Google Scholar] [CrossRef]
- Guan, Y.C.; Luo, F.F.; Lim, G.C.; Hong, M.H.; Zheng, H.Y.; Qi, B. Fabrication of metallic surfaces with long-term superhydrophilic property using one-stop laser method. Mater. Des. 2015, 78, 19–24. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef]
- Cardoso, J.T.; Garcia-Girón, A.; Romano, J.M.; Huerta-Murillo, D.; Jagdheesh, R.; Walker, M.; Dimov, S.S.; Ocaña, J.L. Influence of ambient conditions on the evolution of wettability properties of an IR-, ns-laser textured aluminium alloy. RSC Adv. 2017, 7, 39617–39627. [Google Scholar] [CrossRef] [Green Version]
- Jagdheesh, R.; Diaz, M.; Ocaña, J.L. Bio inspired self-cleaning ultrahydrophobic aluminium surface by laser processing. RSC Adv. 2016, 6, 72933–72941. [Google Scholar] [CrossRef]
- Huerta-Murillo, D.; García-Girón, A.; Romano, J.M.; Cardoso, J.T.; Cordovilla, F.; Walker, M.; Dimov, S.S.; Ocaña, J.L. Wettability modification of laser-fabricated hierarchical surface structures in Ti6Al4V titanium alloy. Appl. Surf. Sci. 2019, 463, 838–846. [Google Scholar] [CrossRef]
- Long, J.; Zhong, M.; Zhang, H.; Fan, P. Superhydrophilicity to superhydrophobicity transition of picosecond laser microstructured aluminum in ambient air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef]
- Jagdheesh, R.; Diaz, M.; Marimuthu, S.; Ocaña, J.L. Hybrid laser and vacuum process for rapid ultrahydrophobic Ti6Al4 V surface formation. Appl. Surf. Sci. 2019, 471, 759–766. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, S.; Fukawa, M.; Hayashi, Y.; Matsumoto, K. Surface OH group governing adsorption properties of metal oxide films. Thin Solid Films 1999, 339, 220–224. [Google Scholar] [CrossRef]
- Jagdheesh, R.; Pathiraj, B.; Karatay, E.; Römer, G.R.B.E.; Huis in’t Veld, A.J. Laser-induced nanoscale superhydrophobic structures on metal surfaces. Langmuir 2011, 27, 8464–8469. [Google Scholar] [CrossRef]
- Bizi-Bandoki, P.; Valette, S.; Audouard, E.; Benayoun, S. Time dependency of the hydrophilicity and hydrophobicity of metallic alloys subjected to femtosecond laser irradiations. Appl. Surf. Sci. 2013, 273, 399–407. [Google Scholar] [CrossRef]
- Lee, B.E.J.; Exir, H.; Weck, A.; Grandfield, K. Characterization and evaluation of femtosecond laser-induced sub-micron periodic structures generated on titanium to improve osseointegration of implants. Appl. Surf. Sci. 2018, 441, 1034–1042. [Google Scholar] [CrossRef]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Wetting behaviour of femtosecond laser textured Ti6Al4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef] [Green Version]
- May, A.; Agarwal, N.; Lee, J.; Lambert, M.; Akkan, C.K.; Nothdurft, F.P.; Aktas, O.C. Laser induced anisotropic wetting on Ti6Al4V surfaces. Mater. Lett. 2015, 138, 21–24. [Google Scholar] [CrossRef]
- Bizi-Bandoki, P.; Benayoun, S.; Valette, S.; Beaugiraud, B.; Audouard, E. Modifications of roughness and wettability properties of metals induced by femtosecond laser treatment. Appl. Surf. Sci. 2011, 257, 5213–5218. [Google Scholar] [CrossRef]
- Lu, J.; Huang, T.; Liu, Z.; Zhang, X.; Xiao, R. Long-term wettability of titanium surfaces by combined femtosecond laser micro/nano structuring and chemical treatments. Appl. Surf. Sci. 2018, 459, 257–262. [Google Scholar] [CrossRef]
- He, A.; Liu, W.; Xue, W.; Yang, H.; Cao, Y. Nanosecond laser ablated copper superhydrophobic surface with tunable ultrahigh adhesion and its renewability with low temperature annealing. Appl. Surf. Sci. 2018, 434, 120–125. [Google Scholar] [CrossRef]
- Milles, S.; Voisiat, B.; Nitschke, M.; Lasagni, A.F. Influence of roughness achieved by periodic structures on the wettability of aluminum using direct laser writing and direct laser interference patterning technology. J. Mater. Process. Technol. 2019, 270, 142–151. [Google Scholar] [CrossRef]
- Zupančič, M.; Može, M.; Gregorčič, P.; Golobič, I. Nanosecond laser texturing of uniformly and non-uniformly wettable micro structured metal surfaces for enhanced boiling heat transfer. Appl. Surf. Sci. 2017, 399, 480–490. [Google Scholar] [CrossRef] [Green Version]
- DIN EN ISO 10993-5:2009-10. Biologische Beurteilung von Medizinprodukten – Teil-5: Prüfungen auf In-vitro-Zytotoxizität (ISO_10993-5:2009); Deutsche Fassung EN_ISO_10993-5:2009; Beuth Verlag GmbH: Berlin, Germany, 2009. [Google Scholar]
- Fadeeva, E.; Deiwick, A.; Chichkov, B.; Schlie-Wolter, S. Impact of laser-structured biomaterial interfaces on guided cell responses. Interface Focus 2014, 4, 20130048. [Google Scholar] [CrossRef]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Cristoforetti, G.; Legnaioli, S.; Palleschi, V.; Tognoni, E.; Benedetti, P.A. Observation of different mass removal regimes during the laser ablation of an aluminium target in air. J. Anal. At. Spectrom. 2008, 23, 1518. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Plomp, A.J.; Su, D.S.; de Jong, K.P.; Bitter, J.H. On the Nature of Oxygen-Containing Surface Groups on Carbon Nanofibers and Their Role for Platinum Deposition—An XPS and Titration Study. J. Phys. Chem. C 2009, 113, 9865–9869. [Google Scholar] [CrossRef]
- Wulser, K.W.; Langell, M.A. Carboxylic acid adsorption on NiO(100) characterized by X-ray photoelectron and high resolution electron energy loss spectroscopies. Catal. Lett. 1992, 15, 39–50. [Google Scholar] [CrossRef]
- Buchholz, M. UHV-FTIRS-Untersuchungen an einkristallinen Oxidoberflächen; KIT Scientific Publishing: Karlsruhe, Germany, 2013. [Google Scholar]
- Strohmeier, B.R. An ESCA method for determining the oxide thickness on aluminum alloys. Surf. Interface Anal. 1990, 15, 51–56. [Google Scholar] [CrossRef]
- Carlson, T.A.; McGuire, G.E. Study of the x-ray photoelectron spectrum of tungsten—Tungsten oxide as a function of thickness of the surface oxide layer. J. Electron Spectrosc. Relat. Phenom. 1972, 1, 161–168. [Google Scholar] [CrossRef]
- Giovambattista, N.; Debenedetti, P.G.; Rossky, P.J. Effect of surface polarity on water contact angle and interfacial hydration structure. J. Phys. Chem. B 2007, 111, 9581–9587. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zheng, H.Y.; Lim, C.P.; Lam, Y.C. Polymer hydrophilicity and hydrophobicity induced by femtosecond laser direct irradiation. Appl. Phys. Lett. 2009, 95, 111110. [Google Scholar] [CrossRef]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Selvarajan, V.; Deshmukh, R.R.; Gao, C. Modification of surface properties of polypropylene (PP) film using DC glow discharge air plasma. Appl. Surf. Sci. 2009, 255, 3965–3971. [Google Scholar] [CrossRef]
- Mildner, J.; Sarpe, C.; Götte, N.; Wollenhaupt, M.; Baumert, T. Emission signal enhancement of laser ablation of metals (aluminum and titanium) by time delayed femtosecond double pulses from femtoseconds to nanoseconds. Appl. Surf. Sci. 2014, 302, 291–298. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Ruud, J.A. Role of hydroxyls in oxide wettability. Langmuir 2010, 26, 1408–1411. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Eng, P.J.; Trainor, T.P.; Brown, G.E., Jr.; Waychunas, G.A.; Newville, M.; Sutton, S.R.; Rivers, M.L. Structure of the hydrated alpha-Al(2)O(3) (0001) surface. Science 2000, 288, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, J.; van Gils, S.; Beentjes, P.C.J.; Terryn, H.; de Wit, J.H.W. Ageing of aluminium oxide surfaces and their subsequent reactivity towards bonding with organic functional groups. Appl. Surf. Sci. 2004, 235, 465–474. [Google Scholar] [CrossRef]
- Tong, S.R.; Wu, L.Y.; Ge, M.F.; Wang, W.G.; Pu, Z.F. Heterogeneous chemistry of monocarboxylic acids on α-Al2O3 at different relative humidities. Atmos. Chem. Phys. 2010, 10, 7561–7574. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X.; Yu, J.C.; Zhong, G.; Han, J.; Zhao, Q. The grain size and surface hydroxyl content of super-hydrophilic TiO2/SiO2 composite nanometer thin films. J. Mater. Sci. Lett. 2001, 20, 1745–1748. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhao, X.; Zhao, Q.; Wang, G. Preparation and characterization of super-hydrophilic porous TiO2 coating films. Mater. Chem. Phys. 2001, 68, 253–259. [Google Scholar] [CrossRef]
- Balchev, I.; Minkovski, N.; Marinova, T.; Shipochka, M.; Sabotinov, N. Composition and structure characterization of aluminum after laser ablation. Mater. Sci. Eng. B 2006, 135, 108–112. [Google Scholar] [CrossRef]
- Dabek-Zlotorzynska, E.; McGrath, M. Determination of low-molecular-weight carboxylic acids in the ambient air and vehicle emissions: A review. Fresenius’ J. Anal. Chem. 2000, 367, 507–518. [Google Scholar] [CrossRef]
- Grönberg, L.; Shen, Y.; Jönsson, J.Å. Ion chromatographic determination of carboxylic in air with on-line liquid membrane pretreatment. J. Chromatogr. A 1993, 655, 207–215. [Google Scholar] [CrossRef]







| Material | Laser Pulse Duration | Cleaning before LT | Atmosphere at LT | Cleaning after LT | Storage after LT | Reference |
|---|---|---|---|---|---|---|
| Al2024 | 15 ns | Isopropanol | Ambient air | Compressed air | Ambient air, polyethylene bags and polystyrene boxes | [27] |
| Ti6Al4V | 30 ns and 310 fs | Ethanol | Ambient air | Compressed air | Ambient air; polyethylene bags | [29] |
| Al | 30 ns | No data (maybe acetone as typical degreasing agent for Al-foils) | Ambient air | No data | Ambient air | [28] |
| Ti6Al4V | 30 ns | No data | Ambient air | No data | High vacuum | [31] |
| Al | 10 ps | Ethanol in US | Ambient air | Ethanol + US, Compressed air | CO2, O2, N2 | [30] |
| Al | 50 ns | acetone, ethanol and deionized water in sequence + US | No data | No data | Ambient Air; Annealing | [32] |
| Cleaning Solvent | Structural Chemical Formula | Purity (%) (* Conductivity (µS/cm)) | Time of Cleaning (min) |
|---|---|---|---|
| No cleaning | - | - | - |
| Pure water |  | 0.055 * | 15 |
| Ethanol |  | ≥99.8 | 15 |
| Acetone |  | ≥99.7 | 15 |
| Isopropanol |  | ≥99.5 | 15 |
| Hexane |  | ≥95 | 15 |
| Specimens | Al0 (at. %) | Al3+ (at. %) | Ti0 (at. %) | Ti4+ (at. %) |
|---|---|---|---|---|
| Reference | 0.7 | 3.2 | 0.7 | 15.2 |
| Structured (no solvent) | 0.7 | 8.8 | 1.0 | 14.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnell, G.; Polley, C.; Bartling, S.; Seitz, H. Effect of Chemical Solvents on the Wetting Behavior Over Time of Femtosecond Laser Structured Ti6Al4V Surfaces. Nanomaterials 2020, 10, 1241. https://doi.org/10.3390/nano10061241
Schnell G, Polley C, Bartling S, Seitz H. Effect of Chemical Solvents on the Wetting Behavior Over Time of Femtosecond Laser Structured Ti6Al4V Surfaces. Nanomaterials. 2020; 10(6):1241. https://doi.org/10.3390/nano10061241
Chicago/Turabian StyleSchnell, Georg, Christian Polley, Stephan Bartling, and Hermann Seitz. 2020. "Effect of Chemical Solvents on the Wetting Behavior Over Time of Femtosecond Laser Structured Ti6Al4V Surfaces" Nanomaterials 10, no. 6: 1241. https://doi.org/10.3390/nano10061241
APA StyleSchnell, G., Polley, C., Bartling, S., & Seitz, H. (2020). Effect of Chemical Solvents on the Wetting Behavior Over Time of Femtosecond Laser Structured Ti6Al4V Surfaces. Nanomaterials, 10(6), 1241. https://doi.org/10.3390/nano10061241