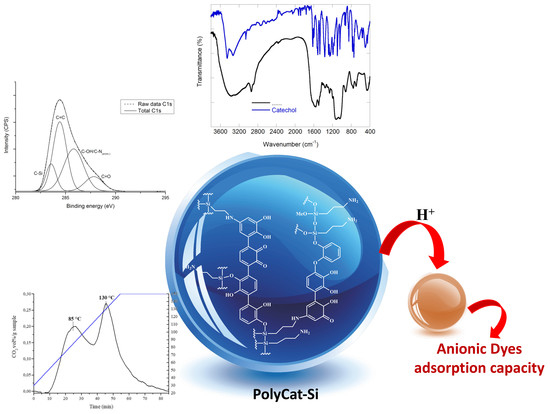
New Mussel Inspired Polydopamine-Like Silica-Based Material for Dye Adsorption
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Massaro, M.; Armetta, F.; Cavallaro, G.; Chillura Martino, D.F.; Gruttadauria, M.; Lazzara, G.; Riela, S.; d’Ischia, M. Effect of halloysite nanotubes filler on polydopamine properties. J. Colloid Interface Sci. 2019, 555, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Gao, T.; Si, S.; Zhang, Z.; Liu, Q.; Zhou, G. Mussel-inspired polymerization of catechol and 1,6-hexamethylenediamine for material-independent surface chemistry. Appl. Surf. Sci. 2020, 507, 145080. [Google Scholar] [CrossRef]
- Zhou, F.; Luo, J.; Song, S.; Wan, Y. Nanostructured polyphenol-mediated coating: A versatile platform for enzyme immobilization and micropollutant removal. Ind. Eng. Chem. Res. 2020, 59, 2708–2717. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Cai, C.; Guo, J.; Fan, H.; Zhu, C.; Dong, H.; Zhao, N.; Xu, J. Mussel inspired modification of polypropylene separators by catechol/polyamine for Li-ion batteries. ACS Appl. Mater. Interface 2014, 6, 5602–5608. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, M.; Paez, J.I.; Avolio, R.; Carpentieri, A.; Panzella, L.; Falco, G.; Pizzo, E.; Errico, M.E.; Napolitano, A.; Del Campo, A.; et al. Multifunctional thin films and coatings from caffeic acid and a cross-linking diamine. Langmuir 2017, 33, 2096–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Y.; Xiao, L.; Cao, Q. Co-polymerization of catechol and polyethylenimine on magnetic nanoparticles for efficient selective removal of anionic dyes from water. Powder Technol. 2017, 310, 24–34. [Google Scholar] [CrossRef]
- Yang, J.; Saggiomo, V.; Velders, A.H.; Cohen Stuart, M.A.; Kamperman, M. Reaction pathways in catechol/primary amine mixtures: A window on crosslinking chemistry. PLoS ONE 2016, 11, e0166490. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Luo, S.; Xu, D.; Liu, S.; Wu, N.; Yao, W.; Zhang, X.; Zheng, L.; Lin, X. Silica-polydopamine hybrids as light-induced oxidase mimics for colorimetric detection of pyrophosphate. Analyst 2020, 145, 424–433. [Google Scholar] [CrossRef]
- Silvestri, B.; Vitiello, G.; Luciani, G.; Calcagno, V.; Costantini, A.; Gallo, M.; Parisi, S.; Paladino, S.; Iacomino, M.; D’Errico, G.; et al. Probing the eumelanin–silica interface in chemically engineered bulk hybrid nanoparticles for targeted subcellular antioxidant protection. ACS Appl. Mater. Interfaces 2017, 9, 37615–37622. [Google Scholar] [CrossRef]
- Wang, Z.; Han, M.; Zhang, J.; He, F.; Xu, Z.; Ji, S.; Peng, S.; Li, Y. Designing preferable functional materials based on the secondary reactions of the hierarchical tannic acid (TA)-aminopropyltriethoxysilane (APTES) coating. Chem. Eng. J. 2019, 360, 299–312. [Google Scholar] [CrossRef]
- Chen, F.; Xing, Y.; Wang, Z.; Zheng, X.; Zhang, J.; Cai, K. Nanoscale polydopamine (pda) meets π–π interactions: An interface-directed coassembly approach for mesoporous nanoparticles. Langmuir 2016, 32, 12119–12128. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Flanagan, D.P.; Orlicki, J.A.; Lenhart, J.L.; Proctor, K.L.; Knorr, D.B. Polydopamine and polydopamine–silane hybrid surface treatments in structural adhesive applications. Langmuir 2018, 34, 1274–1286. [Google Scholar] [CrossRef]
- Fu, Y.; Cai, M.; Zhang, E.; Cao, S.; Ji, P. A novel hybrid polymer network for efficient anticorrosive and antibacterial coatings. Ind. Eng. Chem. Res. 2016, 55, 4482–4489. [Google Scholar] [CrossRef]
- Demey, H.; Barron-Zambrano, J.; Mhadhbi, T.; Miloudi, H.; Yang, Z.; Ruiz, M.; Sastre, A.M. Boron removal from aqueous solutions by using a novel alginate-based sorbent: Comparison with Al2O3 particles. Polymers 2019, 11, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and desorption studies of Pb(II) and Ni(II) from aqueous solutions by a new composite based on alginate and magadiite materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudaoud, N.; Miloudi, H.; Bouazza, D.; Adjdir, M.; Tayeb, A.; Fortuny, A.; Demey, H.; Sastre, A.M. Removal of zinc from aqueous solutions using lamellar double hydroxide materials impregnated with cyanex 272: Characterization and sorption studies. Molecules 2020, 25, 1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Yang, J.; Cohen Stuart, M.A.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Haemers, S.; Koper, G.J.M.; Frens, G. Effect of oxidation rate on cross-linking of mussel adhesive proteins. Biomacromolecules 2003, 4, 632–640. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Bouazizi, N.; Beltrao-Nunes, A.-P.; Launay, F.; Pailleret, A.; Pillier, F.; Bengueddach, A.; Hamacha, R.; Azzouz, A. Mesoporous silica supported amine and amine-copper complex for CO2 adsorption: Detailed reaction mechanism of hydrophilic character and CO2 retention. J. Mol. Struct. 2019, 1191, 175–182. [Google Scholar] [CrossRef]
- Ghomari, K.; Benhamou, A.; Hamacha, R.; Bengueddach, A.; Nousir, S.; Shiao, T.C.; Roy, R.; Azzouz, A. TPD and DSC insights in the basicity of MCM-48-like silica and modified counterparts. Thermochim. Acta 2015, 600, 52–61. [Google Scholar] [CrossRef]
- Hakiki, A.; Boukoussa, B.; Habib Zahmani, H.; Hamacha, R.; Hadj Abdelkader, N.e.H.; Bekkar, F.; Bettahar, F.; Nunes-Beltrao, A.P.; Hacini, S.; Bengueddach, A.; et al. Synthesis and characterization of mesoporous silica SBA-15 functionalized by mono-, di-, and tri-amine and its catalytic behavior towards michael addition. Mater. Chem. Phys. 2018, 212, 415–425. [Google Scholar] [CrossRef]
- Yue, M.B.; Chun, Y.; Cao, Y.; Dong, X.; Zhu, J.H. CO2 capture by as-prepared SBA-15 with an occluded organic template. Adv. Funct. Mater. 2006, 16, 1717–1722. [Google Scholar] [CrossRef]
- Oubaha, M.; Dubois, M.; Murphy, B.; Etienne, P. Structural characterisation of a sol-gel copolymer synthesised from aliphatic and aromatic alkoxysilanes using 29Si-NMR spectroscopy. J. Sol-Gel Sci. Technol. 2006, 38, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Guo, J.; Ma, J.; Shao, L. Highly regenerable alkali-resistant magnetic nanoparticles inspired by mussels for rapid selective dye removal offer high-efficiency environmental remediation. J. Mater. Chem. A 2015, 3, 19960–19968. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Yang, Q.; Chen, H.; Chen, X.; Jiao, T.; Peng, Q. Distinguished Cr(VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism. J. Hazard. Mater. 2018, 342, 732–740. [Google Scholar] [CrossRef]
- Wang, K.; Fu, J.; Wang, S.; Gao, M.; Zhu, J.; Wang, Z.; Xu, Q. Polydopamine-coated magnetic nanochains as efficient dye adsorbent with good recyclability and magnetic separability. J. Colloid Interface Sci. 2018, 516, 263–273. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Mahadwad, O.K.; Parikh, P.A.; Jasra, R.V.; Patil, C. Photocatalytic degradation of reactive black-5 dye using TiO2 impregnated ZSM-5. Bull. Mater. Sci. 2011, 34, 551–556. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, D.; Liu, X.; Pei, X.; Chen, L.; Jin, J. Bio-inspired surface-functionalization of graphene oxide for the adsorption of organic dyes and heavy metal ions with a superhigh capacity. J. Mater. Chem. A 2014, 2, 5034–5040. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Wu, Z.; Wu, Y.; Gao, T.; Yao, J. Efficient removal of methyl orange and alizarin red s from pH-unregulated aqueous solution by the catechol–amine resin composite using hydrocellulose as precursor. ACS Sustain. Chem. Eng. 2017, 5, 1871–1880. [Google Scholar] [CrossRef]
- Wang, C.; Yin, J.; Wang, R.; Jiao, T.; Huang, H.; Zhou, J.; Zhang, L.; Peng, Q. Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Liu, R. Fe3O4@polydopamine and derived Fe3O4@carbon core–shell nanoparticles: Comparison in adsorption for cationic and anionic dyes. Colloids Surf. Physicochem. Eng. Asp. 2017, 522, 260–265. [Google Scholar] [CrossRef]
- Fu, J.; Xin, Q.; Wu, X.; Chen, Z.; Yan, Y.; Liu, S.; Wang, M.; Xu, Q. Selective adsorption and separation of organic dyes from aqueous solution on polydopamine microspheres. J. Colloid Interface Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef] [PubMed]










| Atom | % |
|---|---|
| Si | 7.3 |
| C | 61.4 |
| N | 7.4 |
| O | 23.6 |
| Functional Groups | Abundance (%) a |
|---|---|
| C | |
| C–Si | 12 |
| C=C | 41 |
| C–OH/C–N(arom.) | 36 |
| C=O | 11 |
| N | |
| NH2 | 48 |
| C–N(arom.) | 52 |
| O | |
| C=O | 7 |
| O–Si | 51 |
| C–OH | 42 |
| Material | Qe mg·g−1 | Ref. |
|---|---|---|
| PDA-GO | 30 | [30] |
| Poly(catechol-tetraethylenepentamine-cyanuric chloride)@hydrocellulose (PCEC-C) | 37.2 | [31] |
| PCL/PEO@PDA-45 | 60.2 | [32] |
| Fe3O4@PDA NPs | 2.1 | [33] |
| PDA microspheres | almost zero | [34] |
| PolyCat-Si | 24 | this work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, M.; Campisciano, V.; Viseras Iborra, C.; Liotta, L.F.; Sánchez-Polo, M.; Riela, S.; Gruttadauria, M. New Mussel Inspired Polydopamine-Like Silica-Based Material for Dye Adsorption. Nanomaterials 2020, 10, 1416. https://doi.org/10.3390/nano10071416
Massaro M, Campisciano V, Viseras Iborra C, Liotta LF, Sánchez-Polo M, Riela S, Gruttadauria M. New Mussel Inspired Polydopamine-Like Silica-Based Material for Dye Adsorption. Nanomaterials. 2020; 10(7):1416. https://doi.org/10.3390/nano10071416
Chicago/Turabian StyleMassaro, Marina, Vincenzo Campisciano, César Viseras Iborra, Leonarda F. Liotta, Manuel Sánchez-Polo, Serena Riela, and Michelangelo Gruttadauria. 2020. "New Mussel Inspired Polydopamine-Like Silica-Based Material for Dye Adsorption" Nanomaterials 10, no. 7: 1416. https://doi.org/10.3390/nano10071416
APA StyleMassaro, M., Campisciano, V., Viseras Iborra, C., Liotta, L. F., Sánchez-Polo, M., Riela, S., & Gruttadauria, M. (2020). New Mussel Inspired Polydopamine-Like Silica-Based Material for Dye Adsorption. Nanomaterials, 10(7), 1416. https://doi.org/10.3390/nano10071416