Laboratory Operando XAS Study of Sodium Iron Titanite Cathode in the Li-Ion Half-Cell
Abstract
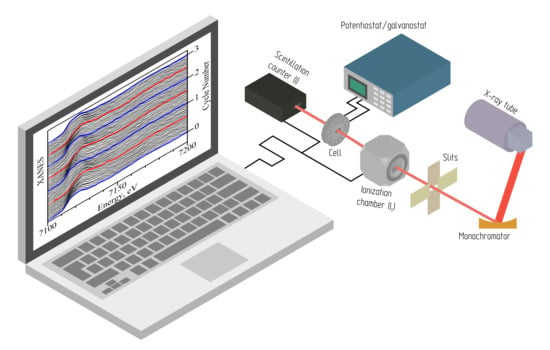
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nadeem, F.; Hussain, S.M.S.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative Review of Energy Storage Systems, Their Roles, and Impacts on Future Power Systems. IEEE Access 2019, 7, 4555–4585. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, J. Demanding energy from carbon. Carbon Energy 2019, 1, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, F.; Zuo, L.; Wang, J.; Yu, N.; Chen, Y.; Zhu, Y.; Huang, Q.; Holze, R.; Wu, Y.; et al. Latest Advances in High-Voltage and High-Energy-Density Aqueous Rechargeable Batteries. Electrochem. Energy Rev. 2020, 1–34. [Google Scholar] [CrossRef]
- Biemolt, J.; Jungbacker, P.; Van Teijlingen, T.; Yan, N.; Rothenberg, G. Beyond Lithium-Based Batteries. Materials 2020, 13, 425. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Qi, X.; Li, L.; Li, W.; Xu, L.; Xie, Y.; Lai, X.; Hu, G.; Peng, Z.; Cao, Y.; et al. Sodium Doping to Enhance Electrochemical Performance of Overlithiated Oxide Cathode Materials for Li-Ion Batteries via Li/Na Ion-Exchange Method. ACS Appl. Mater. Interfaces 2018, 10, 27141–27149. [Google Scholar] [CrossRef]
- Perez, A.J.; Rousse, G.; Tarascon, J.-M. Structural Instability Driven by Li/Na Competition in Na(Li1/3Ir2/3)O2 Cathode Material for Li-Ion and Na-Ion Batteries. Inorg. Chem. 2019, 58, 15644–15651. [Google Scholar] [CrossRef]
- Gerasimova, L.G.; Nikolaev, A.I.; Shchukina, E.S.; Maslova, M.V. Titanite-Containing Mineral Compositions and Their Chemical Treatment with Preparation of Functional Materials. Materials 2020, 13, 1599. [Google Scholar] [CrossRef] [Green Version]
- Voronina, N.; Jo, J.H.; Choi, J.U.; Konarov, A.; Kim, J.; Myung, S.-T. Revealing sodium storage mechanism in lithium titanium phosphate: Combined experimental and theoretical study. J. Power Sources 2020, 455, 227976. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Harks, P.P.R.M.L.; Mulder, F.M.; Notten, P.H.L. In situ methods for Li-ion battery research: A review of recent developments. J. Power Sources 2015, 288, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Kimpton, J.A.; Brand, H.E.A.; Wang, Z.; Chou, S. Solving Key Challenges in Battery Research Using In Situ Synchrotron and Neutron Techniques. Adv. Energy Mater. 2017, 7, 1602831. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.-Y.; Yang, H.; Cai, W.; Liu, S.; Liu, B. In Situ/Operando Characterization Techniques to Probe the Electrochemical Reactions for Energy Conversion. Small Methods 2018, 2, 1700395. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Shen, L.; Zhu, C.; Huang, Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 29. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Zhang, W.; Mao, M.; Wei, Z.; Wang, L.; Cui, C.; Zhu, Y.; Ma, J. Research progress on vanadium-based cathode materials for sodium ion batteries. J. Mater. Chem. A 2018, 6, 8815–8838. [Google Scholar] [CrossRef]
- Broux, T.; Bamine, T.; Simonelli, L.; Stievano, L.; Fauth, F.; Ménétrier, M.; Carlier, D.; Masquelier, C.; Croguennec, L. VIVDisproportionation Upon Sodium Extraction From Na3V2(PO4)2F3 Observed by Operando X-ray Absorption Spectroscopy and Solid-State NMR. J. Phys. Chem. C 2017, 121, 4103–4111. [Google Scholar] [CrossRef]
- Liu, R.; Xu, G.; Li, Q.; Zheng, S.; Zheng, G.; Gong, Z.; Li, Y.; Kruskop, E.; Fu, R.; Chen, Z.; et al. Exploring Highly Reversible 1.5-Electron Reactions (V3+/V4+/V5+) in Na3VCr(PO4)3 Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 43632–43639. [Google Scholar] [CrossRef]
- Liu, R.; Liang, Z.; Xiang, Y.; Zhao, W.; Liu, H.; Chen, Y.; An, K.; Yang, Y. Recognition of V3+/V4+/V5+ Multielectron Reactions in Na3V(PO4)2: A Potential High Energy Density Cathode for Sodium-Ion Batteries. Molecules 2020, 25, 1000. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, J.; Zhang, X.; Ren, Y.; Zuo, P.; Yin, G.; Wang, J. Unravelling the origin of irreversible capacity loss in NaNiO2 for high voltage sodium ion batteries. Nano Energy 2017, 34, 215–223. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Song, Y.; Li, Q.; Liu, Y.; Chen, J.; Xing, X. Understanding the superior sodium-ion storage in a novel Na3.5Mn0.5V1.5(PO4)3 cathode. Energy Storage Mater. 2019, 23, 25–34. [Google Scholar] [CrossRef]
- Wang, D.; Bie, X.; Fu, Q.; Dixon, D.; Bramnik, N.; Hu, Y.S.; Fauth, F.; Wei, Y.; Ehrenberg, H.; Chen, G.; et al. Sodium vanadium titanium phosphate electrode for symmetric sodium-ion batteries with high power and long lifespan. Nat. Commun. 2017, 8, 15888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapovalov, V.V.; Guda, A.A.; Kosova, N.V.; Kubrin, S.P.; Podgornova, O.A.; Aboraia, A.M.; Lamberti, C.; Soldatov, A.V. Laboratory operando Fe and Mn K-edges XANES and Mössbauer studies of the LiFe0.5Mn0.5PO4 cathode material. Radiat. Phys. Chem. 2020, 175, 108065. [Google Scholar] [CrossRef]
- Shukaev, I.L.; Butova, V.V.; Chernenko, S.V.; Pospelov, A.A.; Shapovalov, V.V.; Guda, A.A.; Aboraia, A.M.; Zahran, H.Y.; Yahia, I.S.; Soldatov, A.V. New orthorhombic sodium iron(+2) titanate. Ceram. Int. 2020, 46, 4416–4422. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Martini, A.; Guda, S.A.; Guda, A.A.; Smolentsev, G.; Algasov, A.; Usoltsev, O.; Soldatov, M.A.; Bugaev, A.; Rusalev, Y.; Lamberti, C.; et al. PyFitit: The software for quantitative analysis of XANES spectra using machine-learning algorithms. Comput. Phys. Commun. 2020, 250, 107064. [Google Scholar] [CrossRef]
- Smolentsev, G.; Soldatov, A.V. FitIt: New software to extract structural information on the basis of XANES fitting. Comput. Mater. Sci. 2007, 39, 569–574. [Google Scholar] [CrossRef]
- Klementiev, K.; Chernikov, R. XAFSmass: A program for calculating the optimal mass of XAFS samples. J. Phys. Conf. Ser. 2016, 712, 12008. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bi, X.; Amine, K.; Lu, J. Oxygen-Based Anion Redox for Lithium Batteries. Acc. Chem. Res. 2020, 53, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.J.; Jacquet, Q.; Batuk, D.; Iadecola, A.; Saubanère, M.; Rousse, G.; Larcher, D.; Vezin, H.; Doublet, M.-L.; Tarascon, J.M. Approaching the limits of cationic and anionic electrochemical activity with the Li-rich layered rocksalt Li3IrO4. Nat. Energy 2017, 2, 954–962. [Google Scholar] [CrossRef]
- Hu, E.; Yu, X.; Lin, R.; Bi, X.; Lu, J.; Bak, S.; Nam, K.-W.; Xin, H.L.; Jaye, C.; Fischer, D.A.; et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 2018, 3, 690–698. [Google Scholar] [CrossRef]
- McCalla, E.; Sougrati, M.T.; Rousse, G.; Berg, E.J.; Abakumov, A.; Recham, N.; Ramesha, K.; Sathiya, M.; Dominko, R.; Van Tendeloo, G.; et al. Understanding the Roles of Anionic Redox and Oxygen Release during Electrochemical Cycling of Lithium-Rich Layered Li4FeSbO6. J. Am. Chem. Soc. 2015, 137, 4804–4814. [Google Scholar] [CrossRef]
- Ishiguro, T.; Tanaka, K.; Marumo, F.; Ismail, M.; Hirano, S.; Somiya, S. Freudenbergite. Acta Crystallogr. Sect. B Struct. Commun. 1978, 34, 255–256. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Chen, B.; Zhang, Z.C.; Wu, X.L.; Du, Y.H.; Huang, Y.; Li, B.; Zong, Y.; Wang, J.; Nam, G.-H.; et al. Improved Reversibility of Fe3+/Fe4+Redox Couple in Sodium Super Ion Conductor Type Na3Fe2(PO4)3 for Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1605694. [Google Scholar] [CrossRef]
- Seidler, G.T.; Mortensen, D.R.; Remesnik, A.J.; Pacold, J.I.; Ball, N.A.; Barry, N.; Styczinski, M.; Hoidn, O.R. A laboratory-based hard x-ray monochromator for high-resolution x-ray emission spectroscopy and x-ray absorption near edge structure measurements. Rev. Sci. Instrum. 2014, 85, 113906. [Google Scholar] [CrossRef]
- Ditter, A.S.; Jahrman, E.P.; Bradshaw, L.R.; Xia, X.; Pauzauskie, P.J.; Seidler, G.T. A mail-in and user facility for X-ray absorption near-edge structure: The CEI-XANES laboratory X-ray spectrometer at the University of Washington. J. Synchrotron Radiat. 2019, 26, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Jahrman, E.P.; Pellerin, L.A.; Ditter, A.S.; Bradshaw, L.R.; Fister, T.T.; Polzin, B.J.; Trask, S.E.; Dunlop, A.R.; Seidler, G.T. Laboratory-Based X-ray Absorption Spectroscopy on a Working Pouch Cell Battery at Industrially-Relevant Charging Rates. J. Electrochem. Soc. 2019, 166, A2549–A2555. [Google Scholar] [CrossRef]
- Yoon, W.-S.; Balasubramanian, M.; Chung, K.Y.; Yang, X.-Q.; McBreen, J.; Grey, C.P.; Fischer, D.A. Investigation of the charge compensation mechanism on the electrochemically Li-ion deintercalated Li1-xCo1/3Ni1/3Mn1/3O2 electrode system by combination of soft and hard X-ray absorption spectroscopy. J. Am. Chem. Soc. 2005, 127, 17479–17487. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kato, D.; Arai, H.; Tanida, H.; Mori, T.; Orikasa, Y.; Uchimoto, Y.; Ohta, T.; Ogumi, Z. Novel spectro-electrochemical cell for in situ/operando observation of common composite electrode with liquid electrolyte by X-ray absorption spectroscopy in the tender X-ray region. Rev. Sci. Instrum. 2014, 85, 084103. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Z.-X.; Yang, W. Redox Mechanism in Na-Ion Battery Cathodes Probed by Advanced Soft X-Ray Spectroscopy. Front. Chem. 2020, 8, 816. [Google Scholar] [CrossRef]
- Saubanère, M.; Yahia, M.B.; Lebègue, S.; Doublet, M.L. An intuitive and efficient method for cell voltage prediction of lithium and sodium-ion batteries. Nat. Commun. 2014, 5, 5559. [Google Scholar] [CrossRef] [Green Version]






| Species | NaxFe0.45Ti1.55O4 | LixFe0.45Ti1.55O4 | Reference Values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | 0 | 0.25 | 0.5 | 0.75 | 1 | 0 | 0.25 | 0.5 | 0.75 | 1 | |||
| Ti | 9.56 | 9.57 | 9.58 | 9.57 | 9.63 | 9.56 | 9.56 | 9.56 | 9.56 | 9.59 | Ti3+ | 9.93 | |
| Fe | 6.25 | 6.21 | 6.21 | 6.44 | 6.44 | 6.20 | 6.19 | 6.24 | 6.46 | 6.60 | Ti4+ | 9.55 | |
| O | 7.13 | 7.19 | 7.24 | 7.27 | 7.29 | 7.14 | 7.20 | 7.25 | 7.28 | 7.31 | Fe2+ | 6.61 | |
| Fe3+ | 6.25 | ||||||||||||
| O2− | 7.31 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapovalov, V.; Guda, A.; Butova, V.; Shukaev, I.; Soldatov, A. Laboratory Operando XAS Study of Sodium Iron Titanite Cathode in the Li-Ion Half-Cell. Nanomaterials 2021, 11, 156. https://doi.org/10.3390/nano11010156
Shapovalov V, Guda A, Butova V, Shukaev I, Soldatov A. Laboratory Operando XAS Study of Sodium Iron Titanite Cathode in the Li-Ion Half-Cell. Nanomaterials. 2021; 11(1):156. https://doi.org/10.3390/nano11010156
Chicago/Turabian StyleShapovalov, Victor, Alexander Guda, Vera Butova, Igor Shukaev, and Alexander Soldatov. 2021. "Laboratory Operando XAS Study of Sodium Iron Titanite Cathode in the Li-Ion Half-Cell" Nanomaterials 11, no. 1: 156. https://doi.org/10.3390/nano11010156
APA StyleShapovalov, V., Guda, A., Butova, V., Shukaev, I., & Soldatov, A. (2021). Laboratory Operando XAS Study of Sodium Iron Titanite Cathode in the Li-Ion Half-Cell. Nanomaterials, 11(1), 156. https://doi.org/10.3390/nano11010156