Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications
Abstract
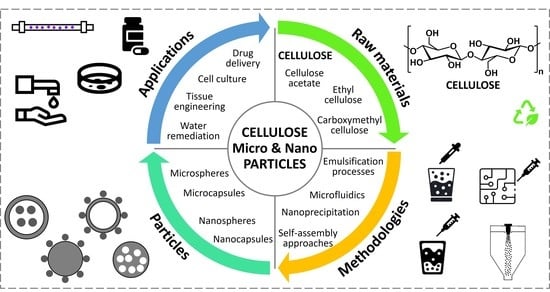
:1. Introduction
2. Cellulose Fundamentals
3. Overview of Spherical Particles Fabrication
4. Production of Spherical Cellulose-Based Microparticles
4.1. Emulsification Processes
4.2. Microfluidics
4.3. Other Methodologies
5. Production of Spherical Cellulose-Based Nanoparticles
5.1. Nanoprecipitation
5.2. Emulsification Processes
5.3. Other Methodologies
6. Applications of Spherical Cellulose-Based Micro and Nanoparticles
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Fang, L.; Wang, L.; Yao, Y.; Zhang, J.; Wu, X.; Li, X.; Wang, H.; Zhang, X.; Gong, X.; Chang, J. Micro- and nano-carrier systems: The non-invasive and painless local administration strategies for disease therapy in mucosal tissues. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 153–171. [Google Scholar] [CrossRef]
- Pang, L.; Gao, Z.; Feng, H.; Wang, S.; Wang, Q. Cellulose based materials for controlled release formulations of agrochemicals: A review of modifications and applications. J. Control. Release 2019, 316, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Badenes, S.M.; Fernandes, T.G.; Rodrigues, C.A.V.; Diogo, M.M.; Cabral, J.M.S. Microcarrier-based platforms for in vitro expansion and differentiation of human pluripotent stem cells in bioreactor culture systems. J. Biotechnol. 2016, 234, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.Q.; Vilela, C.; Santos, H.A.; Silvestre, A.J.D.; Freire, C.S.R. Recent trends on the development of systems for cancer diagnosis and treatment by microfluidic technology. Appl. Mater. Today 2020, 18, 100450. [Google Scholar] [CrossRef]
- Leitgeb, M.; Knez, Ž.; Vasić, K. Micro- and Nanocarriers for Immobilization of Enzymes. In Micro and Nanotechnologies for Biotechnology; Stanciu, S.G., Ed.; IntechOpen: London, UK, 2016; pp. 21–58. [Google Scholar]
- Ngomsik, A.F.; Bee, A.; Draye, M.; Cote, G.; Cabuil, V. Magnetic nano- and microparticles for metal removal and environmental applications: A review. Comptes Rendus Chim. 2005, 8, 963–970. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Liu, J.; Jiang, P.; You, S.; Ding, N.; Guo, Q.; Lin, F. Applications of nanomaterials for heavy metal removal from water and soil: A review. Sustainability 2021, 13, 713. [Google Scholar] [CrossRef]
- Terna, A.D.; Elemike, E.E.; Mbonu, J.I.; Osafile, O.E.; Ezeani, R.O. The future of semiconductors nanoparticles: Synthesis, properties and applications. Mater. Sci. Eng. B 2021, 272, 115363. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Carbon nanotubes: A potential material for energy conversion and storage. Prog. Energy Combust. Sci. 2018, 64, 219–253. [Google Scholar] [CrossRef]
- Tarelho, J.P.G.; Soares dos Santos, M.P.; Ferreira, J.A.F.; Ramos, A.; Kopyl, S.; Kim, S.O.; Hong, S.; Kholkin, A. Graphene-based materials and structures for energy harvesting with fluids—A review. Mater. Today 2018, 21, 1019–1041. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles: A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Jaspart, S.; Piel, G.; Delattre, L.; Evrard, B. Solid lipid microparticles: Formulation, preparation, characterisation, drug release and applications. Expert Opin. Drug Deliv. 2005, 2, 75–87. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Biomed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchera, A.; Bettini, R. Polysaccharide nanoparticles for oral controlled drug delivery: The role of drug–polymer and interpolymer interactions. Expert Opin. Drug Deliv. 2020, 17, 1345–1359. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- United Nations Transforming Our World: The 2030 Agenda for sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 3 June 2021).
- Stanisz, M.; Klapiszewski, Ł.; Jesionowski, T. Recent advances in the fabrication and application of biopolymer-based micro- and nanostructures: A comprehensive review. Chem. Eng. J. 2020, 397, 125409. [Google Scholar] [CrossRef]
- Zhao, X.F.; Winter, W.T. Cellulose/cellulose-based nanospheres: Perspectives and prospective. Ind. Biotechnol. 2015, 11, 34–43. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Ko, J.A.; Park, H.J.; Hwang, S.J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Pham, D.T.; Tiyaboonchai, W. Fibroin nanoparticles: A promising drug delivery system. Drug Deliv. 2020, 27, 431–448. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, F.; Ali, A.; Kircher, M.F.; Pal, S. DNA Nanostructures and DNA-Functionalized Nanoparticles for Cancer Theranostics. Adv. Sci. 2020, 7, 2001669. [Google Scholar] [CrossRef] [PubMed]
- Willem de Vries, J.; Schnichels, S.; Hurst, J.; Strudel, L.; Gruszka, A.; Kwak, M.; Bartz-Schmidt, K.U.; Spitzer, M.S.; Herrmann, A.; Shu, Y.; et al. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Biomaterials 2014, 66, 74–89. [Google Scholar] [CrossRef] [Green Version]
- Index Mundi Wood Pulp Monthly Price. Available online: https://www.indexmundi.com/commodities/?commodity=wood-pulp¤cy=eur (accessed on 8 May 2021).
- CellufineTM. Available online: https://www.jnc-corp.co.jp/fine/en/cellufine/ (accessed on 5 August 2021).
- Macroporous Bead Cellulose, MT. Available online: https://www.iontosorb.cz/pages/bead.htm (accessed on 5 August 2021).
- CytoporeTM Macroporous Microcarriers. Available online: https://www.cytivalifesciences.com/en/dk/shop/cell-culture-and-fermentation/microcarriers/cytopore-1-microcarriers-dry-powder-p-05928#tech-spec-table (accessed on 24 August 2021).
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- CelluForce NCC®. Available online: https://www.celluforce.com/en/products/cellulose-nanocrystals/ (accessed on 6 August 2021).
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Gericke, M.; Trygg, J.; Fardim, P. Functional cellulose beads: Preparation, characterization, and applications. Chem. Rev. 2013, 113, 4812–4836. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2020, 6, 124–144. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Lameirinhas, N.S.; Guedes, G.; Silva, G.H.R. da; Oskoei, P.; Spirk, S.; Oliveira, H.; Duarte, I.F.; Vilela, C.; Freire, C.S.R. Cellulose nanocrystals/chitosan-based nanosystems: Synthesis, characterization, and cellular uptake on breast cancer cells. Nanomaterials 2021, 11, 2057. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Frollini, E.; Scott, J. Cellulose Nanoparticles: Volume 1: Chemistry and Fundamentals; Thakur, V.K., Frollini, E., Scott, J., Eds.; Royal Society of Chemistry: London, UK, 2021; ISBN 978-1-78801-793-0. [Google Scholar]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Production and characteristics of cellulose from different sources. In Cellulose Derivatives; Heinze, T., El Seoud, O.A., Koschella, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–38. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and properties. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2015; Volume 271, pp. 1–52. [Google Scholar]
- Dufresne, A. Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Philos. Trans. R. Soc. A 2018, 376, 20170040. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.B.; de Azeredo, H.M.C.; Aouada, F.A.; Feitosa, J.P.A.; Rosa, M.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Modification of textiles for functional applications. In Fundamentals of Natural Fibres and Textiles (The Textile Institute Book Series); Mondal, M.I.H., Ed.; Woodhead Publishing, Elsevier Ltd.: Cambridge, UK, 2021; pp. 303–365. ISBN 9780128214831. [Google Scholar]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Selyanchyn, O.; Selyanchyn, R.; Lyth, S.M. A Review of Proton Conductivity in Cellulosic Materials. Front. Energy Res. 2020, 8, 596164. [Google Scholar] [CrossRef]
- Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible nanocellulose/lignosulfonates ion conducting separators for polymer electrolyte fuel cells. Nanomaterials 2020, 10, 1713. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Martins, M.A.; Lucas, J.M.F.; Vilela, C.; Sales, A.J.M.; Costa, L.C.; Marques, P.A.A.P.; Freire, C.S.R. High-electroconductive nanopapers based on nanocellulose and copper nanowires: A new generation of flexible and sustainable electrical materials. ACS Appl. Mater. Interfaces 2020, 12, 34208–34216. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Grishkewich, N.; Tam, K.C. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Figueira, P.; Fabre, E.; Pinto, R.J.B.; Pereira, M.E.; Silvestre, A.J.D.; Marrucho, I.M.; Vilela, C.; Freire, C.S.R. Dual nanofibrillar-based bio-sorbent films composed of nanocellulose and lysozyme nanofibrils for mercury removal from spring waters. Carbohydr. Polym. 2020, 238, 116210. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose bio-based composites for food packaging. Nanomaterials 2020, 10, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Bastante, C.C.; Silva, N.H.C.S.; Cardoso, L.C.; Serrano, C.M.; Martínez de la Ossa, E.J.; Freire, C.S.R.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.M.; Veiga, M.; Antunes, F.; Pintado, M.M.; Sèbe, G.; Coma, V.; et al. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Almeida, T.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial nanocellulose toward green cosmetics: Recent progresses and challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Freire, C.S.R.; Neto, C.P. Do bacterial cellulose membranes have potential in drug-delivery systems? Expert Opin. Drug Deliv. 2014, 11, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.P.F.; Silva, A.C.Q.; Bastos, V.; Oliveira, H.; Pinto, R.J.B.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Nanocellulose-based patches loaded with hyaluronic acid and diclofenac towards aphthous stomatitis treatment. Nanomaterials 2020, 10, 628. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Y.; Chen, J.Y.; Tong, X.M.; Mei, J.G.; Chen, Y.F.; Mou, X.Z. Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol. Lett. 2020, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Osch, D.J.G.P.; Kollau, L.J.B.M.; Van Den Bruinhorst, A.; Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.S.; Lopes, A.M.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Gluconacetobacter sacchari: An efficient bacterial cellulose cell-factory. Carbohydr. Polym. 2011, 86, 1417–1420. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; Vilela, C.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial cellulose-based nanocomposites: Roadmap for innovative materials. In Nanocellulose Polymer Composites; Thakur, V.K., Ed.; Scrivener Publishing LLC: Salem, MA, USA, 2015; pp. 17–64. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Figueiredo, A.R.P.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Development and applications of cellulose nanofibers based polymer composites. In Advanced Composite Materials: Properties and Applications; Bafekrpour, E., Ed.; De Gruyter Open: Berlin, Germany, 2017; pp. 1–65. [Google Scholar]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Special Issue: Advanced Biopolymer-Based Nanocomposites and Hybrid Materials. Materials 2021, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Valente, B.F.A.; Silvestre, A.J.D.; Neto, C.P.; Vilela, C.; Freire, C.S.R. Effect of the micronization of pulp fibers on the properties of green composites. Molecules 2021, 26, 5594. [Google Scholar] [CrossRef]
- Liebert, T. Cellulose solvents-remarkable history, bright future. In ACS Symposium Series; Liebert, T.F., Heinze, T.J., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1033, pp. 3–54. [Google Scholar]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, I.; Inamoto, M.; Matsui, T.; Saito, M.; Okajima, K. Studies on structure of cuprammonium cellulose I. A circular dichroism study on the dissolved state of cellulose in cuprammonium solution. Polym. J. 1995, 27, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Alexandridis, P.; Ghasemi, M.; Furlani, E.P.; Tsianou, M. Solvent processing of cellulose for effective bioresource utilization. Curr. Opin. Green Sustain. Chem. 2018, 14, 40–52. [Google Scholar] [CrossRef]
- Wan, Y.; An, F.; Zhou, P.; Li, Y.; Liu, Y.; Lu, C.; Chen, H. Regenerated cellulose I from LiCl·DMAc solution. Chem. Commun. 2017, 53, 3595–3597. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Jedvert, K.; Heinze, T. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Tassaing, T.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Detailed understanding of the DBU/CO2 switchable solvent system for cellulose solubilization and derivatization. ACS Sustain. Chem. Eng. 2018, 6, 1496–1503. [Google Scholar] [CrossRef] [Green Version]
- Clough, M.T. Organic electrolyte solutions as versatile media for the dissolution and regeneration of cellulose. Green Chem. 2017, 19, 4754–4768. [Google Scholar] [CrossRef] [Green Version]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O. Recent Advances in Solvents for the Dissolution, Shaping and Derivatization of Cellulose: Quaternary Ammonium Electrolytes and their Solutions in Water and Molecular Solvents. Molecules 2018, 23, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef]
- Kim, M.H.; An, S.; Won, K.; Kim, H.J.; Lee, S.H. Entrapment of enzymes into cellulose-biopolymer composite hydrogel beads using biocompatible ionic liquid. J. Mol. Catal. B Enzym. 2012, 75, 68–72. [Google Scholar] [CrossRef]
- Druel, L.; Kenkel, A.; Baudron, V.; Buwalda, S.; Budtova, T. Cellulose Aerogel Microparticles via Emulsion-Coagulation Technique. Biomacromolecules 2020, 21, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Jimmy, F.B.; Pang, S.C. Size controlled fabrication of cellulose nanoparticles for drug delivery applications. J. Drug Deliv. Sci. Technol. 2018, 43, 262–266. [Google Scholar] [CrossRef]
- Shen, J.; Shafiq, M.; Ma, M.; Chen, H. Synthesis and surface engineering of inorganic nanomaterials based on microfluidic technology. Nanomaterials 2020, 10, 1177. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Braz, A.L.; Ahmed, I. Manufacturing processes for polymeric micro and nanoparticles and their biomedical applications. AIMS Bioeng. 2017, 4, 46–72. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Sheth, T.; Seshadri, S.; Prileszky, T.; Helgeson, M.E. Multiple nanoemulsions. Nat. Rev. Mater. 2020, 5, 214–228. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo effect”: Application to drug delivery devices. Adv. Drug Deliv. Rev. 2014, 71, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Bernard, J.; Ganachaud, F. Nanoprecipitation as a simple and straightforward process to create complex polymeric colloidal morphologies. Adv. Colloid Interface Sci. 2021, 294, 102474. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Fung, S.; Zheng, Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin. B 2019, 9, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.K.; Lee, D. Biopolymer microparticles prepared by microfluidics for biomedical applications. Small 2020, 16, 1903736. [Google Scholar] [CrossRef]
- Kung, C.T.; Gao, H.; Lee, C.Y.; Wang, Y.N.; Dong, W.; Ko, C.H.; Wang, G.; Fu, L.M. Microfluidic synthesis control technology and its application in drug delivery, bioimaging, biosensing, environmental analysis and cell analysis. Chem. Eng. J. 2020, 399, 125748. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Ferreira, M.P.A.; Correia, A.; Hirvonen, J.; Santos, H.A. Microfluidics as a cutting-edge technique for drug delivery applications. J. Drug Deliv. Sci. Technol. 2016, 34, 76–87. [Google Scholar] [CrossRef]
- Boskovic, D.; Loebbecke, S. Synthesis of polymer particles and capsules employing microfluidic techniques. Nanotechnol. Rev. 2014, 3, 27–38. [Google Scholar] [CrossRef]
- Kashani, S.Y.; Afzalian, A.; Shirinichi, F.; Moraveji, M.K. Microfluidics for core-shell drug carrier particles—A review. RSC Adv. 2020, 11, 229–249. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Hensel, A.; Brandner, J.J.; Zhang, K.; Du, X.; Yang, Y. A review on emulsification via microfluidic processes. Front. Chem. Sci. Eng. 2020, 14, 350–364. [Google Scholar] [CrossRef]
- Vilela, C.; Figueiredo, A.R.P.; Silvestre, A.J.D.; Freire, C.S.R. Multilayered materials based on biopolymers as drug delivery systems. Expert Opin. Drug Deliv. 2017, 14, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Ma, W.; Wu, H.; Huang, L.; Chen, L.; Takahara, A. Fabrication of cellulose based superhydrophobic microspheres for the production of magnetically actuatable smart liquid marbles. J. Bioresour. Bioprod. 2017, 2, 110–115. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, J.; Zhang, L. Drugs adsorption and release behavior of collagen/bacterial cellulose porous microspheres. Int. J. Biol. Macromol. 2019, 140, 196–205. [Google Scholar] [CrossRef]
- Simões, M.G.; Coimbra, P.; Carreira, A.S.; Figueiredo, M.M.; Gil, M.H.; Simões, P.N. Eugenol-loaded microspheres incorporated into textile substrates. Cellulose 2020, 27, 4109–4121. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Oh, Y.; Yun, J.; Yoo, E.; Jung, D.; Oh, K.K.; Lee, S.H. Cellulose/biopolymer/Fe3O4 hydrogel microbeads for dye and protein adsorption. Cellulose 2020, 27, 2757–2773. [Google Scholar] [CrossRef]
- Yu, J.; Huang, T.-R.; Lim, Z.H.; Luo, R.; Pasula, R.R.; Liao, L.-D.; Lim, S.; Chen, C.-H. Production of Hollow Bacterial Cellulose Microspheres Using Microfluidics to Form an Injectable Porous Scaffold for Wound Healing. Adv. Healthc. Mater. 2016, 5, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Miki, N. Hydrogel fiber cultivation method for forming bacterial cellulose microspheres. Micromachines 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Babayekhorasani, F.; Spicer, P.T. Soft bacterial cellulose microcapsules with adaptable shapes. Biomacromolecules 2019, 20, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, W.; Ren, M.; Ren, X. Fabrication of porous cellulose microspheres with controllable structures by microfluidic and flash freezing method. Mater. Lett. 2020, 262, 127193. [Google Scholar] [CrossRef]
- Liu, Y.; Nambu, N.O.; Taya, M. Cell-laden microgel prepared using a biocompatible aqueous two-phase strategy. Biomed. Microdevices 2017, 19, 55. [Google Scholar] [CrossRef]
- Levin, D.; Saem, S.; Osorio, D.A.; Cerf, A.; Cranston, E.D.; Moran-Mirabal, J.M. Green templating of ultraporous cross-linked cellulose nanocrystal microparticles. Chem. Mater. 2018, 30, 8040–8051. [Google Scholar] [CrossRef]
- Kaufman, G.; Mukhopadhyay, S.; Rokhlenko, Y.; Nejati, S.; Boltyanskiy, R.; Choo, Y.; Loewenberg, M.; Osuji, C.O. Highly stiff yet elastic microcapsules incorporating cellulose nanofibrils. Soft Matter 2017, 13, 2733–2737. [Google Scholar] [CrossRef]
- Yeap, E.W.Q.; Acevedo, A.J.; Khan, S.A. Microfluidic extractive crystallization for spherical drug/drug-excipient microparticle production. Org. Process Res. Dev. 2019, 23, 375–381. [Google Scholar] [CrossRef]
- Yeap, E.W.Q.; Ng, D.Z.L.; Prhashanna, A.; Somasundar, A.; Acevedo, A.J.; Xu, Q.; Salahioglu, F.; Garland, M.V.; Khan, S.A. Bottom-up structural design of crystalline drug-excipient composite microparticles via microfluidic droplet-based processing. Cryst. Growth Des. 2017, 17, 3030–3039. [Google Scholar] [CrossRef]
- Pepicelli, M.; Binelli, M.R.; Studart, A.R.; Rühs, P.A.; Fischer, P. Self-grown bacterial cellulose capsules made through emulsion templating. ACS Biomater. Sci. Eng. 2021, 7, 3221–3228. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Caputo, T.; Ambrosio, L.; Caserta, S.; Calcagnile, P.; Demitri, C. Mono- and bi-phasic cellulose acetate micro-vectors for anti-inflammatory drug delivery. Pharmaceutics 2019, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wu, W.; Zhang, X.; Meng, X.; Tong, G.; Deng, Y. Temperature-sensitive poly-NIPAm modified cellulose nanofibril cryogel microspheres for controlled drug release. Cellulose 2016, 23, 415–425. [Google Scholar] [CrossRef]
- Deng, J.-R.; Zhao, C.-L.; Wu, Y.-X. Antibacterial and pH-responsive quaternized hydroxypropyl cellulose-g-poly(THF-co-epichlorohydrin) graft copolymer: Synthesis, characterization and properties. Chin. J. Polym. Sci. 2020, 38, 704–714. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, H.; Dou, J.; Tong, G.; Deng, Y. Cellulose nanofibril based-aerogel microreactors: A high efficiency and easy recoverable W/O/W membrane separation system. Sci. Rep. 2017, 7, 40096. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Lee, S.H.; Kim, J.C. Spray-dried microparticles composed of carboxylated cellulose nanofiber and cysteamine and their oxidation-responsive release property. Colloid Polym. Sci. 2020, 298, 157–167. [Google Scholar] [CrossRef]
- Wang, F.J.; Lu, F.S.; Cui, M.; Shao, Z.Q. Biocompatible microcapsule of carboxymethyl cellulose/chitosan as drug carrier. Adv. Mater. Res. 2015, 1118, 227–236. [Google Scholar] [CrossRef]
- Paulraj, T.; Riazanova, A.V.; Svagan, A.J. Bioinspired capsules based on nanocellulose, xyloglucan and pectin—The influence of capsule wall composition on permeability properties. Acta Biomater. 2018, 69, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Al-Omar, M.S.; El-Readi, M.Z.; Alhowail, A.H.; Aldubayan, M.A.; Abdellatif, A.A.H. Formulation of ethyl cellulose microparticles incorporated pheophytin a isolated from suaeda vermiculata for antioxidant and cytotoxic activities. Molecules 2019, 24, 1501. [Google Scholar] [CrossRef] [Green Version]
- Nethaji, R.; Shanub, S.; Manikandan, P.; Surendiran, N.S.; Babu, G. Preparation and characterization of ethyl cellulose microspheres containing diclofenac sodium. Int. J. Res. Pharm. Nano Sci. 2016, 5, 224–234. [Google Scholar]
- Wang, S.; Yang, Y.; Lu, A.; Zhang, L. Construction of cellulose/ZnO composite microspheres in NaOH/zinc nitrate aqueous solution via one-step method. Cellulose 2019, 26, 557–568. [Google Scholar] [CrossRef]
- Pei, Y.; Wu, X.; Xu, G.; Sun, Z.; Zheng, X.; Liu, J.; Tang, K. Tannin-immobilized cellulose microspheres as effective adsorbents for removing cationic dye (Methylene Blue) from aqueous solution. J. Chem. Technol. Biotechnol. 2017, 92, 1276–1284. [Google Scholar] [CrossRef]
- Sha, Q.; Wu, Y.; Wang, C.; Sun, B.; Zhang, Z.; Zhang, L.; Lin, Y.; Liu, X. Cellulose microspheres-filled pipet tips for purification and enrichment of glycans and glycopeptides. J. Chromatogr. A 2018, 1569, 8–16. [Google Scholar] [CrossRef] [PubMed]
- OBrien, J.C.; Torrente-Murciano, L.; Mattia, D.; Scott, J.L. Continuous production of cellulose microbeads via membrane emulsification. ACS Sustain. Chem. Eng. 2017, 5, 5931–5939. [Google Scholar] [CrossRef] [Green Version]
- Omura, T.; Suzuki, T.; Minami, H. Preparation of cellulose particles with a hollow structure. Langmuir 2020, 36, 14076–14082. [Google Scholar] [CrossRef]
- Murakami, M.; Matsumoto, A.; Watanabe, C.; Kurumado, Y.; Takama, M. Fabrication of porous ethyl cellulose microspheres based on the acetone-glycerin-water ternary system: Controlling porosity via the solvent-removal mode. Drug Discov. Ther. 2015, 9, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Abbaspoor, S.; Ashrafi, A.; Salehi, M. Synthesis and characterization of ethyl cellulose micro/nanocapsules using solvent evaporation method. Colloid Polym. Sci. 2018, 296, 1509–1514. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, T.; Turng, L.S. Aerogel microspheres based on cellulose nanofibrils as potential cell culture scaffolds. Cellulose 2017, 24, 2791–2799. [Google Scholar] [CrossRef]
- Lin, W.H.; Jana, S.C. Analysis of porous structures of cellulose aerogel monoliths and microparticles. Microporous Mesoporous Mater. 2021, 310, 110625. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Yu, K.; Meng, H.; Zheng, Y.; Peng, J.; Lu, S.; Liu, X.; Xie, Y.; Qiao, K. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 2018, 171, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Gao, F.; Zeng, D.; Peng, C.; Chen, Y.; Li, M. Synthesis of Ag–Fe3O4 nanoparticles supported on polydopamine-functionalized porous cellulose acetate microspheres: Catalytic and antibacterial applications. Cellulose 2018, 25, 4771–4782. [Google Scholar] [CrossRef]
- Huang, A.; Li, X.; Liang, X.; Zhang, Y.; Hu, H.; Yin, Y.; Huang, Z. Solid-phase synthesis of cellulose acetate butyrate as microsphere wall materials for sustained release of emamectin benzoate. Polymers 2018, 10, 1381. [Google Scholar] [CrossRef] [Green Version]
- Lee, H. Bin; Yoon, S.Y.; Singh, B.; Oh, S.H.; Cui, L.; Yan, C.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Oral immunization of FMDV vaccine using pH-sensitive and mucoadhesive thiolated cellulose acetate phthalate microparticles. Tissue Eng. Regen. Med. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M. Preparation and adsorption studies of phosphorylated cellulose microspheres. Cellul. Chem. Technol. 2017, 51, 23–34. [Google Scholar]
- Wu, Q.X.; Lin, D.Q.; Yao, S.J. Fabrication and formation studies on single-walled CA/NaCS-WSC microcapsules. Mater. Sci. Eng. C 2016, 59, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Muhaimin; Bodmeier, R. Effect of solvent type on preparation of ethyl cellulose microparticles by solvent evaporation method with double emulsion system using focused beam reflectance measurement. Polym. Int. 2017, 66, 1448–1455. [Google Scholar] [CrossRef]
- Setyono, D.; Valiyaveettil, S. Use of porous cellulose microcapsules for water treatment. RSC Adv. 2015, 5, 83286–83294. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, X.J.; Li, S.J.; Ying, X.Y.; Hu, J.B.; Xu, X.L.; Kang, X.Q.; You, J.; Du, Y.Z. Preparation of ethyl cellulose microspheres for sustained release of sodium bicarbonate. Iran. J. Pharm. Res. 2019, 18, 556–568. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Inclusion of hydroxytyrosol in ethyl cellulose microparticles: In vitro release studies under digestion conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Božič, M.; Elschner, T.; Tkaučič, D.; Bračič, M.; Hribernik, S.; Stana Kleinschek, K.; Kargl, R. Effect of different surface active polysaccharide derivatives on the formation of ethyl cellulose particles by the emulsion-solvent evaporation method. Cellulose 2018, 25, 6901–6922. [Google Scholar] [CrossRef] [Green Version]
- Dhand, A.P.; Poling-Skutvik, R.; Osuji, C.O. Simple production of cellulose nanofibril microcapsules and the rheology of their suspensions. Soft Matter 2021, 17, 4517–4524. [Google Scholar] [CrossRef] [PubMed]
- Carrick, C.; Larsson, P.A.; Brismar, H.; Aidun, C.; Wågberg, L. Native and functionalized micrometre-sized cellulose capsules prepared by microfluidic flow focusing. RSC Adv. 2014, 4, 19061. [Google Scholar] [CrossRef]
- Kim, S.T.; Cho, S.; Song, M.; Chang, S.T. Microfluidic Synthesis of Microfibers Based on Regeneration of Cellulose from Ionic Liquids. Polymer 2015, 39, 588–592. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials—Physics and Chemistry—New Edition; IntechOpen: London, UK, 2018; pp. 9–35. [Google Scholar]
- Dai, L.; Liu, K.F.; Si, C.L.; He, J.; Lei, J. Du; Guo, L.Q. A novel self-assembled targeted nanoparticle platform based on carboxymethylcellulose co-delivery of anticancer drugs. J. Mater. Chem. B 2015, 3, 6605–6617. [Google Scholar] [CrossRef]
- Dewangan, A.K.; Mazumder, S.; Perumal, Y.; Chopra, K.; Mazumder, S. Preparation, characterization and anti-inflammatory effects of curcumin loaded carboxymethyl cellulose acetate butyrate nanoparticles on adjuvant induced arthritis in rats. J. Drug Deliv. Sci. Technol. 2017, 41, 269–279. [Google Scholar] [CrossRef]
- Hayden, D.R.; Imhof, A.; Velikov, K.P. Biobased nanoparticles for broadband UV protection with photostabilized UV Filters. ACS Appl. Mater. Interfaces 2016, 8, 32655–32660. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, F.; Jin, L.; Liu, B.; Mao, Y.; Liu, Y.; Huang, J. Preparation of spherical nanocellulose from waste paper by aqueous NaOH/thiourea. Cellulose 2019, 26, 5177–5185. [Google Scholar] [CrossRef]
- Dai, L.; Si, C.L. Cellulose-graft-poly(methyl methacrylate) nanoparticles with high biocompatibility for hydrophobic anti-cancer drug delivery. Mater. Lett. 2017, 207, 213–216. [Google Scholar] [CrossRef]
- Peng, X.; Liu, P.; Pang, B.; Yao, Y.; Wang, J.; Zhang, K. Facile fabrication of pH-responsive nanoparticles from cellulose derivatives via Schiff base formation for controlled release. Carbohydr. Polym. 2019, 216, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Al Hakkak, J.; Grigsby, W.J.; Kathirgamanathan, K.; Edmonds, N.R. Generation of Spherical Cellulose Nanoparticles from Ionic Liquid Processing via Novel Nonsolvent Addition and Drying. Adv. Mater. Sci. Eng. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ho, B.K.; Chin, S.F.; Pang, S.C. pH-responsive carboxylic cellulose acetate nanoparticles for controlled release of penicillin G. J. Sci. Adv. Mater. Devices 2020, 5, 224–232. [Google Scholar] [CrossRef]
- Vidal-Romero, G.; Zambrano-Zaragoza, M.L.; Martínez-Acevedo, L.; Leyva-Gómez, G.; Mendoza-Elvira, S.E.; Quintanar-Guerrero, D. Design and evaluation of pH-dependent nanosystems based on cellulose acetate phthalate, nanoparticles loaded with chlorhexidine for periodontal treatment. Pharmaceutics 2019, 11, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshy, K.S.; Snigdha, S.; George, A.; Kalarikkal, N.; Pothen, L.A.; Thomas, S. Core–shell nanoparticles of carboxy methyl cellulose and compritol-PEG for antiretroviral drug delivery. Cellulose 2017, 24, 4759–4771. [Google Scholar] [CrossRef]
- Volmajer Valh, J.; Vajnhandl, S.; Škodič, L.; Lobnik, A.; Turel, M.; Vončina, B. Effects of Ultrasound Irradiation on the Preparation of Ethyl Cellulose Nanocapsules Containing Spirooxazine Dye. J. Nanomater. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Pan-In, P.; Wongsomboon, A.; Kokpol, C.; Chaichanawongsaroj, N.; Wanichwecharungruang, S. Depositing α-mangostin nanoparticles to sebaceous gland area for acne treatment. J. Pharmacol. Sci. 2015, 129, 226–232. [Google Scholar] [CrossRef] [Green Version]
- El-Habashy, S.E.; Allam, A.N.; El-Kamel, A.H. Ethyl cellulose nanoparticles as a platform to decrease ulcerogenic potential of piroxicam: Formulation and in vitro/in vivo evaluation. Int. J. Nanomed. 2016, 11, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin—Assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef]
- Tirado, D.F.; Palazzo, I.; Scognamiglio, M.; Calvo, L.; Della Porta, G.; Reverchon, E. Astaxanthin encapsulation in ethyl cellulose carriers by continuous supercritical emulsions extraction: A study on particle size, encapsulation efficiency, release profile and antioxidant activity. J. Supercrit. Fluids 2019, 150, 128–136. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, X.; Li, M.; Zhao, X.; Zeng, X.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Stability of soluble dialdehyde cellulose and the formation of hollow microspheres: Optimization and characterization. ACS Sustain. Chem. Eng. 2019, 7, 2151–2159. [Google Scholar] [CrossRef]
- Yan, G.; Feng, Y.; Gao, Z.; Zeng, X.; Hong, W.; Liu, W.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Stable and biocompatible cellulose-based CaCO3 microspheres for tunable pH-responsive drug delivery. ACS Sustain. Chem. Eng. 2019, 7, 19824–19831. [Google Scholar] [CrossRef]
- Kumari, S.; Ram, B.; Kumar, D.; Ranote, S.; Chauhan, G.S. Nanoparticles of oxidized-cellulose synthesized by green method. Mater. Sci. Energy Technol. 2018, 1, 22–28. [Google Scholar] [CrossRef]
- Sirviö, J.A. Fabrication of regenerated cellulose nanoparticles by mechanical disintegration of cellulose after dissolution and regeneration from a deep eutectic solvent. J. Mater. Chem. A 2019, 7, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem. Eng. J. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Deng, X.-Y.; Shen, W.-H.; Jia, M.-Y. Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Chen, X.Q. Preparation and characterization of spherical cellulose nanocrystals with high purity by the composite enzymolysis of pulp fibers. Bioresour. Technol. 2019, 291, 121842. [Google Scholar] [CrossRef]
- Yan, C.F.; Yu, H.Y.; Yao, J.M. One-step extraction and functionalization of cellulose nanospheres from lyocell fibers with cellulose II crystal structure. Cellulose 2015, 22, 3773–3788. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhang, H.; Song, M.L.; Zhou, Y.; Yao, J.; Ni, Q.Q. From cellulose nanospheres, nanorods to nanofibers: Various aspect ratio induced nucleation/reinforcing effects on polylactic acid for robust-barrier food packaging. ACS Appl. Mater. Interfaces 2017, 9, 43920–43938. [Google Scholar] [CrossRef]
- Elumalai, R.; Patil, S.; Maliyakkal, N.; Rangarajan, A.; Kondaiah, P.; Raichur, A.M. Protamine-carboxymethyl cellulose magnetic nanocapsules for enhanced delivery of anticancer drugs against drug resistant cancers. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 969–981. [Google Scholar] [CrossRef]
- Cordt, C.; Meckel, T.; Geissler, A.; Biesalski, M. Entrapment of hydrophobic biocides into cellulose acetate nanoparticles by nanoprecipitation. Nanomaterials 2020, 10, 2447. [Google Scholar] [CrossRef]
- Abbaspoor, S.; Ashrafi, A.; Abolfarsi, R. Development of self-healing coatings based on ethyl cellulose micro/nano-capsules. Surf. Eng. 2019, 35, 273–280. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Guo, W.; Ma, F.; Sun, S.; Zhou, Q. Anti-solvents tuning cellulose nanoparticles through two competitive regeneration routes. Cellulose 2018, 25, 4513–4523. [Google Scholar] [CrossRef]
- Topel, S.D.; Balcioglu, S.; Ateş, B.; Asilturk, M.; Topel, Ö.; Ericson, M.B. Cellulose acetate encapsulated upconversion nanoparticles—A novel theranostic platform. Mater. Today Commun. 2021, 26, 101829. [Google Scholar] [CrossRef]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Saidane, D.; Perrin, E.; Cherhal, F.; Guellec, F.; Capron, I. Some modification of cellulose nanocrystals for functional Pickering emulsions. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150139. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S. Material design of nanocellulose/polymer composites via Pickering emulsion templating. Polym. J. 2021, 53, 103–109. [Google Scholar] [CrossRef]
- Rescignano, N.; Fortunati, E.; Armentano, I.; Hernandez, R.; Mijangos, C.; Pasquino, R.; Kenny, J.M. Use of alginate, chitosan and cellulose nanocrystals as emulsion stabilizers in the synthesis of biodegradable polymeric nanoparticles. J. Colloid Interface Sci. 2015, 445, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedzior, S.A.; Gabriel, V.A.; Dubé, M.A.; Cranston, E.D. Nanocellulose in emulsions and heterogeneous water-based polymer systems: A review. Adv. Mater. 2021, 33, 2002404. [Google Scholar] [CrossRef]
- Liu, G.; Lin, G.; Lin, X.; Zhou, H.; Chen, H.; Hao, L.; Zhou, X. Enzyme and pH dual-responsive avermectin nano-microcapsules for improving its efficacy. Environ. Sci. Pollut. Res. 2019, 26, 25107–25116. [Google Scholar] [CrossRef]
- Chen, H.; Lin, G.; Zhou, H.; Zhou, X.; Xu, H.; Huang, S. Preparation of avermectin/grafted CMC nanoparticles and their sustained release performance. J. Polym. Environ. 2018, 26, 2945–2953. [Google Scholar] [CrossRef]
- Hao, L.; Lin, G.; Lian, J.; Chen, L.; Zhou, H.; Chen, H.; Xu, H.; Zhou, X. Carboxymethyl cellulose capsulated zein as pesticide nano-delivery system for improving adhesion and anti-UV properties. Carbohydr. Polym. 2020, 231, 115725. [Google Scholar] [CrossRef]
- Movagharnezhad, N.; Moghadam, P.N. Hexamethylene diamine/carboxymethyl cellulose grafted on magnetic nanoparticles for controlled drug delivery. Polym. Bull. 2017, 74, 4645–4658. [Google Scholar] [CrossRef]
- Shafiei-Irannejad, V.; Rahimi, M.; Zarei, M.; Dinparast-isaleh, R.; Bahrambeigi, S.; Alihemmati, A.; Shojaei, S.; Ghasemi, Z.; Yousefi, B. Polyelectrolyte carboxymethyl cellulose for enhanced delivery of doxorubicin in MCF7 breast cancer cells: Toxicological evaluations in mice model. Pharm. Res. 2019, 36, 68. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Kuznetsov, V.A.; Lavlinskaya, M.S. Synthesis of graft copolymers of carboxymethyl cellulose and N,N-dimethylaminoethyl methacrylate and their study as Paclitaxel carriers. Polym. Bull. 2021, 78, 2975–2992. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Yang, H.; Bian, L.; Chang, C.; Zhang, L. Biocompatible cellulose-based supramolecular nanoparticles driven by host–guest interactions for drug delivery. Carbohydr. Polym. 2020, 237, 116114. [Google Scholar] [CrossRef]
- Lu, A.; Petit, E.; Jelonek, K.; Orchel, A.; Kasperczyk, J.; Wang, Y.; Su, F.; Li, S. Self-assembled micelles prepared from bio-based hydroxypropyl methyl cellulose and polylactide amphiphilic block copolymers for anti-tumor drug release. Int. J. Biol. Macromol. 2020, 154, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Hinman, J.J.; Suslick, K.S. Nanostructured Materials Synthesis Using Ultrasound. Top. Curr. Chem. 2017, 375, 12. [Google Scholar] [CrossRef]
- Beaumont, M.; Nypelö, T.; König, J.; Zirbs, R.; Opietnik, M.; Potthast, A.; Rosenau, T. Synthesis of redispersible spherical cellulose II nanoparticles decorated with carboxylate groups. Green Chem. 2016, 18, 1465–1468. [Google Scholar] [CrossRef]
- Beaumont, M.; Rosenfeldt, S.; Tardy, B.L.; Gusenbauer, C.; Khakalo, A.; Nonappa; Opietnik, M.; Potthast, A.; Rojas, O.J.; Rosenau, T. Soft cellulose II nanospheres: Sol–gel behaviour, swelling and material synthesis. Nanoscale 2019, 11, 17773–17781. [Google Scholar] [CrossRef]
- Solin, K.; Beaumont, M.; Rosenfeldt, S.; Orelma, H.; Borghei, M.; Bacher, M.; Opietnik, M.; Rojas, O.J. Self-Assembly of Soft Cellulose Nanospheres into Colloidal Gel Layers with Enhanced Protein Adsorption Capability for Next-Generation Immunoassays. Small 2020, 16, 2004702. [Google Scholar] [CrossRef] [PubMed]
- Eivazi, A.; Medronho, B.; Lindman, B.; Norgren, M. On the development of all-cellulose capsules by vesicle-templated layer-by-layer assembly. Polymers 2021, 13, 589. [Google Scholar] [CrossRef]
- Ibrahim, I.; Lim, H.N.; Huang, N.M. Cellulose acetate beads modified with cadmium sulfide and Methylene blue for adsorbent-assisted photoelectrochemical detection of copper(II) ions. Microchim. Acta 2019, 186, 452. [Google Scholar] [CrossRef]
- Cai, Y.; Yuan, F.; Wang, X.; Sun, Z.; Chen, Y.; Liu, Z.; Wang, X.; Yang, S.; Wang, S. Synthesis of core–shell structured Fe3O4@carboxymethyl cellulose magnetic composite for highly efficient removal of Eu(III). Cellulose 2017, 24, 175–190. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Javanbakht, S.; Behzadi Nia, S.; Namazi, H. Carboxymethyl cellulose/mesoporous magnetic graphene oxide as a safe and sustained ibuprofen delivery bio-system: Synthesis, characterization, and study of drug release kinetic. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124662. [Google Scholar] [CrossRef]
- Gaihre, B.; Jayasuriya, A.C. Fabrication and characterization of carboxymethyl cellulose novel microparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Zeng, J.; Liu, S.; Zhang, L. An effective and recyclable adsorbent for the removal of heavy metal ions from aqueous system: Magnetic chitosan/cellulose microspheres. Bioresour. Technol. 2015, 194, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate-carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef] [PubMed]








| Cellulosic Substrate | General Features | Diameter (μm) | Application | Ref. |
|---|---|---|---|---|
| Bamboo pulp | Aqueous phase: cellulose in NaOH/urea/H2O (7:12:81 wt.%) Oil phase: paraffin oil/Span® 80 Emulsion type: w/o Modified with magnetic nanoparticles and poly(DOPAm-co-PFOEA) a posteriori | ~30 (microcapsule) | – | [112] |
| BNC | Aqueous phase: gelatin/K. xylinus bacterium Oil phase: corn oil/Span® 80 Emulsion type: w/o | ~10 (microsphere) | – | [117] |
| BNC | Aqueous phase: oxidized BNC in [C1mim][Cl]/collagen/polystyrene templates/TWEEN® 80 Oil phase: n-hexadecane/Span® 80 Emulsion type: w/o | 8–12 (microsphere) | Cell culture (MC3T3-E1 cells) Drug delivery (model drug: BSA) | [113] |
| BNC | Aqueous phase: DHYL-DBC/chitosan in acetic acid solution Oil phase: Paraffin oil Emulsion type: w/o | ~450 (microsphere) | Cell culture (BMSCs cells) | [144] |
| BNC | Aqueous phase: 2% v/v bacterial solution Oil phase: decane Emulsion type: w/o | 350 (microcapsule) | – | [125] |
| CA | Aqueous phase: PVA in water Oil phase: CA in ethyl acetate/eugenol Emulsion type: o/w | ~1.3 (microsphere) | Functional textiles (active agent: eugenol) | [114] |
| CA | Aqueous phase: Span® 80/TWEEN® 80 in water Oil phase: CA in DMF/DCM Emulsion type: w/o | ~5 (microsphere) | Catalysis | [145] |
| CAB | Aqueous phase: PVA in water Oil phase: CAB in chloroform/EB Emulsion type: o/w | 70–150 (microsphere) | Pesticide delivery (EB) | [146] |
| CAB | Aqueous phase: PVA in water Oil phase: CAB in ethyl acetate/eugenol Emulsion type: o/w | ~2.2 (microsphere) | Functional textiles (active agent: eugenol) | [114] |
| CAP | Aqueous phase: PVA in water Oil phase: CAP in chloroform + ethanol/eugenol Emulsion type: o/w | ~20 (microsphere) | Functional textiles (active agent: eugenol) | [114] |
| CAP (thiolated) | Aqueous phase: w1: M5BT/Pluronic® F-127, w2: PVA in water Oil phase: CAP in DCM and ethyl acetate/ethanol Emulsion type: w/o/w | ~3.20 (microsphere) | Oral vaccination (M5BT subunit vaccine) | [147] |
| CNFs | Aqueous phase: CNFs/PVA in deionized water/glutaraldehyde Oil phase: Toluene/Span® 80 Emulsion type: w/o (Crosslinking with glutaraldehyde) | 94.5 ± 16.1 503.9 ± 73.5 (microsphere) | Cell culture (NIH 3T3 cells) | [142] |
| Cotton linter pulp | Aqueous phase: cotton pulp dissolved in NaOH/zinc nitrate aqueous solution Oil phase: Isooctane/Span® 80 Emulsion type: w/o (In situ precipitation of ZnO nanoparticles) | ~60 (microsphere) | – | [135] |
| Cotton linter pulp | Aqueous phase: cellulose in NaOH/urea/ H2O + tannins from Areca catechu Oil phase: Paraffin oil/Span® 80/TWEEN® 80 Emulsion type: w/o (Microcapsules crosslinked with epichlorohydrin) | 408 ± 15 (microsphere) | Water remediation (organic dye: methylene blue) | [136] |
| Cotton linter pulp | Aqueous phase: Cellulose in NaOH/urea/H2O Oil phase: Paraffin oil/Span® 80 Emulsion type: w/o | ~12 (microsphere) | HILIC stationary phase | [137] |
| CP | Aqueous phase: CP in NaOH solution Oil phase: 1,2-dichloroethane with CAB Emulsion type: w/o (Microsphere crosslinked with epichlorohydrin) | 10–20 (microsphere) | Water remediation (organic dyes: methylene blue, rhodamine 6G) | [148] |
| CS | Aqueous phase: CS/alginate/chitosan Oil phase: Isooctane/Span® 80+TWEEN® 80 Emulsion type: w/o | 62.4 ± 13.9 (microcapsule) | – | [149] |
| EC | Aqueous phase: PEG/SDS in nitric acid aqueous solution Oil phase: EC in benzene+ ethanol/linseed oil Emulsion type: o/w | 400 (microcapsule) | – | [141] |
| EC | Aqueous phase: methylcellulose in water Oil phase: EC in chloroform/Pheo-a Emulsion type: o/w | 149–163 (microsphere) | – | [133] |
| EC | Aqueous phase: glycerin in water/PVA Oil phase: EC in acetone Emulsion type: o/w | 13.7 ± 0.5 (microsphere) | – | [140] |
| EC | Aqueous phase: w1: water, w2: PVA in water Oil phase: EC in DCM or DCM/methanol or chloroform or ethyl acetate Emulsion type: w/o/w | 60–133 (microsphere) | – | [150] |
| EC | Aqueous phase: w1: PVA in water, w2: PEI in water Oil phase: EC in chloroform/PVP/SDS Emulsion type: w/o/w (Microcapsules crosslinked with glutaraldehyde) | 5–30 (microcapsule) | Water remediation (metal nanoparticles: Ag and Au NPs) | [151] |
| EC | Aqueous phase: PVA in water Oil phase: EC in chloroform+ ethanol/eugenol Emulsion type: o/w | ~11.5 (microsphere) | Functional textiles (active agent: eugenol) | [114] |
| EC | Aqueous phase: Distilled water/TWEEN® 80 Oil phase: EC in ethyl acetate or ethyl acetate/acetone Emulsion type: o/w | 382.4 ± 0.6 to 998.1 ± 0.8 (microsphere) | Drug delivery (NSAID: diclofenac) | [134] |
| EC | Aqueous phase: sodium bicarbonate aqueous solution Oil phase: o1: EC in acetonitrile/TWEEN® 80 o2: Soybean oil/Span® 80 Emulsion type: s/o/o | 280–340 (microsphere) | Drug delivery (model drug: sodium bicarbonate) | [152] |
| EC | Aqueous phase: w1: hydroxytyrosol in double distilled water, w2: PVA in water Oil phase: EC in DCM Emulsion type: w/o/w | 156.6 ± 6.9 to 304.0 ± 16.0 (microcapsule) | Drug release (model drug: hydroxytyrosol) | [153] |
| MCC | Aqueous phase: MCC/Fe3O4/chitosan, κ-carrageenan, lignin or starch in [C2mim][Ac] Oil phase: Pump oil/Span® 80 Emulsion type: w/o | 39–62 (microsphere) | Protein immobilization (pepsin, BSA, lysozyme) Water remediation (organic dyes: crystal violet, methyl orange) | [115] |
| MCC | Aqueous phase: MCC in NaOH/urea/H2O Oil phase: o1: cellulose solution/paraffin oil, o2: nonsolvent+Span® 80/paraffin oil Emulsion type: w/o/o | 5.4 ± 1.8 to 20.9 ± 8.9 (microsphere) | – | [93] |
| MCC | Dispersed phase: MCC in [C2mim][Ac]/ DMSO Continuous phase: sunflower oil/Span® 80 Emulsion type: w/o | 17–135 (microsphere) | – | [138] |
| MCC | Dispersed phase: MCC/[Bmim]Cl/DMF Continuous phase: cyclohexane/Hypermer 1599™ + TWEEN® 80 Emulsion type: o/o | 23 ± 19 to 54 ± 36 (microsphere) | Drug delivery (analgesic drug: acetaminophen) | [143] |
| Cellulosic Substrate | General Features | Diameter (μm) | Application | Ref. |
|---|---|---|---|---|
| BNC | Dispersed phase: alginate microcapsules/agarose/G. xylinus/culture medium Continuous phase: HFE-7500 fluorocarbon oil/ Krytox™ modified with PEG QD = 0.1–0.5 µL min−1; QC = 5 µL min−1 Cross-junction droplet generator | ~50 (microcapsule) | Cell culture (PC-9 cells) Wound healing (rat skin model) | [116] |
| BNC | Dispersed phase: gelatin + bacteria Continuous phase: corn oil with Span® 80 QD = NR; QC = 50–1000 µL min−1 Co-flow microfluidic device | ~250–1000 (microsphere) | – | [117] |
| BNC | Dispersed phase: A. xylinum/ culture medium Continuous phase: hydrogenated castor oil QD = 1.2 µL min−1; QC = 12 µL min−1 Co-flow microfluidic device | >100 (microcapsule) | – | [118] |
| BNC | Dispersed phase: pure medium (inner phase) and bacterial suspension (middle phase) Continuous phase: decane with surfactant (Span® 85 or phosphatidylcholine) QD = 200 μL h−1 (inner phase) and 800 μL h−1 (middle phase); QC = ~333 µL min−1 Flow-focusing device for transient double emulsions | 80−500 (microcapsule) | – | [125] |
| CA | Dispersed phase: CA in DMA, DMF or DMSO Continuous phase: n-hexane/ Span® 80 QD = 10 µL min−1; QC = 100–400 µL min−1 T-junction microfluidic device | 270–750 (microsphere) | Water remediation (organic dye: Congo red) | [119] |
| CMC | Dispersed phase: Ph-CMC/DEX/HRP Continuous phase: PEG/PEG and H2O2 QD = NR; QC = NR Co-flow microfluidic device | 65–111 (microcapsule) | Cell culture (HepG2 cells) | [120] |
| CNCs | Dispersed phase: sCNCs or aCNCs/hCNCs Continuous phase: soybean oil/PGPR QD = 1.6–4 µL min−1; QC = 2–5 µL min−1 T-junction droplet microfluidic device | 30–110 (microcapsule) | – | [121] |
| CNFs | Dispersed phase: CNFs water suspension Continuous phase: MADQUAT-co-BTA in toluene QD = NR; QC = NR Glass capillary microfluidic device | 303 ± 3.4 (microcapsule) | – | [122] |
| CNFs (TEMPO oxidized) | Dispersed phase: aqueous CNFs suspension Continuous phase: oleylamine/toluene solution QD = 10−40 µL min−1; QC = 200−400 µL min−1 T-junction microfluidic device | 25–200 (microcapsule) | – | [155] |
| Dissolving cellulose pulp | Dispersed phase: octane (inner phase) and cellulose solution of LiCl/DMA (middle phase) Continuous phase: silicone oil QD = 10 μL h−1 (inner phase) and 60 μL h−1 (middle phase); QC = 2,000 μL h−1 Microfluidic flow focusing device | 88 μm (microcapsule) | Drug delivery (model drug: FITC-dextran) | [156] |
| EC | Dispersed phase: EC/ROY or EC/carbamazepine in dichloromethane Continuous phase: aqueous PVA solution QD = NR; QC = NR Glass capillary microfluidic device (counter-flow configuration) | 150−300 (microsphere) | Drug delivery (model drug: ROY; anticonvulsant drug: carbamazepine) | [124] |
| EC | Dispersed phase: EC/naproxen in ethyl acetate Continuous phase: aqueous PVA solution QD = 200−500 µL min−1; QC = 50−120 µL min−1 Microfluidic T-junction device | 55–220 (microsphere) | Drug delivery (NSAID: naproxen) | [123] |
| Cellulosic Substrate | General Features | Diameter (μm) | Application | Ref. |
|---|---|---|---|---|
| LAYER-BY-LAYER ASSEMBLY | ||||
| CMC | Methodology: (CMC/CH)16 bilayers on a MF template | ~2.15 (microcapsule) | Drug delivery (antibiotic: tetracycline) | [131] |
| QA-CNFs | Methodology: (CNFs/XyG/CNFs/AP)2CNFs /XyG bilayers on a CaCO3 template | 16 ± 4 (microcapsule) | Drug delivery Cell culture (HEK 293T cells) | [132] |
| SPRAY-ASSISTED TECHNIQUES | ||||
| CA | Solvent solution: acetone/bi-distilled water Flow rate: 0.5–1.5 mL h−1 | 287 ± 76 to 1248 ± 120 (microcapsule) | Drug delivery (NSAID: ketoprofen lysinate) | [126] |
| CNFs | Solvent solution: water Modification: CNFs crosslinked with PA/EP resin; microspheres crosslinked with NIPAm Flow rate: NR | 50–150 (microsphere) | Drug delivery (anticancer drug: 5-fluorouracil) | [127] |
| HPC | Solvent solution: THF Modification: HPC-g-QCP (THF-co-ECH) Flow rate: NR | 3–3.3 (microsphere) | Drug delivery (NSAID: ibuprofen) | [128] |
| t-CNFs | Solvent solution: water Modification: crosslinking with PA/EP resin Flow rate: NR | 2–7 (microsphere) | Water remediation (metal ion: Cu2+) | [129] |
| t-CNFs | Solvent solution: water/cysteamine/FITC-dextran Flow rate: NR | 12.1–13.8 (microsphere) | Drug delivery (model drug: FITC-dextran) | [130] |
| Cellulosic Substrate | General Features | Diameter (nm) | Application | Ref. |
|---|---|---|---|---|
| CA | Solution: CA in acetone Antisolvent: water | ~300 (nanosphere) | Biocide coatings (4-hexylresorcinol, triclosan) | [184] |
| CA | Solution: CA and UCNPs dispersed in a mixture of dichloromethane and acetone Antisolvent: water with SDS | 320 ± 5 (nanocapsule) | Drug delivery (anticancer drug: DOX) | [187] |
| CMC | Solution: FA-PEG-CMC-BA/HCPT in DMSO Antisolvent: PBS solution (pH 7.4) | 186 ± 11 (nanocapsule) | Drug delivery (anticancer drugs: BA, hydroxycamptothecine) | [159] |
| CMCAB | Solution: CMCAB/curcumin in THF Antisolvent: water | 166.5 ± 4.2 (nanosphere) | Drug delivery (anti-inflammatory drug: curcumin) | [160] |
| Cotton fibres | Solution: cotton dissolved in NaOH/urea/thiourea (8/8/6.5 wt.%)/MB Antisolvent: ethanol | 70–365 (nanosphere) | Drug delivery (model drug: methylene blue) | [94] |
| EC | Solution: EC/α-tocopherol or oxybenzone or avobenzone or octinoxate in ethanol Antisolvent: water | ~50 (nanocapsule) | Cosmetics (UV-filters in sunscreens) | [161] |
| Kraft paper/ wastepaper cellulose | Solution: paper waste dissolved in NaOH/urea/thiourea (8/8/6.5 wt.%) Antisolvent: water | ~50 (nanosphere) | Transistors and batteries | [162] |
| MCC | Solution: CE-g-PMMA/BA in DMSO Antisolvent: PBS | ~120 (nanocapsule) | Drug delivery (anticancer drug: BA) | [163] |
| MCC | Solution: DAC (obtained by cellulose oxidation with sodium periodate)/oleylamine/AERhB in DMF Antisolvent: water | 152.1 ± 0.9 156.3 ± 1.0 (nanocapsule) | Drug delivery (model drug: AERhB) | [164] |
| MCC | Solution: MCC dissolved in [C2mim][Ac] Antisolvent: acetonitrile | 100–400 (nanosphere) | – | [165] |
| Cellulose fibres (from paper waste) | Solution: carboxylic CA (obtained via TEMPO oxidation and acetylation) in ultrapure water Antisolvent: ethanol | 70–100 (nanosphere) | Drug delivery (antibiotic: penicillin G) | [166] |
| Cellulosic Substrate | General Features | Diameter (nm) | Application | Ref. |
|---|---|---|---|---|
| CAP | Aqueous phase: PVA in water Oil phase: CAP in methyl ethyl ketone /eugenol oil/CHX (for nanocapsules) and CAP in methyl ethyl ketone/CHX (for the control nanospheres) Emulsion type: o/w | ~248 (nanosphere) 290–324 (nanocapsule) | Drug delivery (antiseptic drug: CHX) | [167] |
| CMC | Aqueous phase: w1: PEG in water and w2: AZT/CMC in water Oil phase: compritol in DCM Emulsion type: w/o/w | 162 ± 44 (nanocapsule) | Drug delivery (antiretroviral drug: AZT) | [168] |
| EC | Aqueous phase: PVA in water Oil phase: spirooxazine dye/EC in DCM Emulsion type: o/w | 193–404 (nanocapsule) | – | [169] |
| EC | Aqueous phase: PEG/SDS in nitric acid aqueous solution Oil phase: EC in ethanol+benzene/linseed oil/n-decane Emulsion type: o/w | 33–473 (nanocapsule) | Anticorrosion coatings | [141,185] |
| EC | Aqueous phase: water Oil phase: EC/MC in ethanol/α-mangostin Emulsion type: w/o | 436 ± 11 (nanosphere) | Drug delivery (antiacne drug: α-mangostin) | [170] |
| EC | Aqueous phase: water/PVA or P188 or CA25 Oil phase: EC in ethyl acetate Emulsion type: o/w | 165 ± 44 to 474 ± 66 (nanosphere) | Drug delivery (NSAID: piroxicam) | [171] |
| EC | Aqueous phase: PVA in water Oil phase: EC in ethyl acetate Emulsion type: o/w | 147 ± 2 (nanosphere) | Drug delivery (corticosteroid drug: dexamethason) | [172] |
| EC | Aqueous phase: ethyl acetate-saturated water/TWEEN® 80 Oil phase: EC in ethyl acetate/astaxanthin Emulsion type: o/w | 161 ± 8 to 733 ± 7 (nanocapsule) | Delivery of bioactive compounds (carotenoid pigment: astaxanthin) | [173] |
| Cellulosic Substrate | General Features | Diameter (nm) | Application | Ref. |
|---|---|---|---|---|
| MECHANICAL TREATMENTS | ||||
| Bamboo pulp | Methodology: high-pressure homogenization followed by oxidation and aging | ~15–35 (nanocapsule) | Drug delivery (hypolipidemic drug: lovastatin) | [175] |
| Cellulose from pine needles | Methodology: cellulose oxidation with TEMPO radical and sodium periodate followed by sonication | <30 (nanosphere) | Drug delivery (anticancer drug: DOX) | [176] |
| Cellulose dissolving pulp (softwood) and MCC | Methodology: mechanical disintegration of the fibres after dissolution and regeneration of cellulose from a DES | 5.6 ± 1.4 5.8 ± 1.4 (nanosphere) | Reinforcement agents | [177] |
| CHEMICAL AND/OR ENZIMATIC TREATMENTS | ||||
| Cotton linter | Methodology: acid hydrolysis followed by lipase catalysed esterification with 3-MPA | 45–75 (nanosphere) | Water remediation (metal ion: Hg2+) | [178] |
| Bleached Kraft eucalyptus pulp | Methodology: enzymatic hydrolysis | 15–40 (nanosphere) | – | [179,180] |
| Lyocell fibres | Methodology: acid hydrolysis followed by the one-pot Fischer esterification with formic acid | 19–29 (nanosphere) | – | [181] |
| Lyocell fibres | Methodology: mixed acid hydrolysis (HCOOH and HCl) of the fibres followed by ultrasonic irradiation | 27.0 ± 1.2 (nanosphere) | Nucleation/reinforcing agent in films for food packaging | [182] |
| SELF-ASSEMBLY PROCESSES | ||||
| CMC | Methodology: graft polymerization of CMC with DMDAAC (CMC-g-PDMDAAC) and encapsulation of avermectin via electrostatic interactions | ~100–150 (nanocapsule) | Pesticide delivery (avermectin) | [193] |
| CMC | Methodology: graft polymerization of CMC with methyl methacrylate (CMC-g-PMMA), butyl acrylate (CMC-g-PBA) or styrene (CMC-g-PS), followed by emulsion to prepare avermectin/grafted polymer nanoparticles | ~230 ~180 230–260 (nanocapsule) | Pesticide delivery (avermectin) | [194] |
| CMC | Methodology: graft polymerization of CMC and DMDAAC (CMC-g-PDMDAAC) followed by electrostatic assembly with P-Zein and encapsulation of avermectin | 360 (nanocapsule) | Pesticide delivery (avermectin) | [195] |
| CMC | Methodology: shell of CMC modified with hexamethylenediamine coated on a core of Fe3O4 nanoparticle | 70–120 (nanocapsule) | Drug delivery (anticancer drug: DOX) | [196] |
| CMC | Methodology: graft polymerization of CMC with ImIL (CMC-g-PIL) followed by coating on a core of Fe3O4 nanoparticle | 39.2 ± 8.4 (nanocapsule) | Drug delivery (anticancer drug: DOX) | [197] |
| CMC | Methodology: graft polymerization of CMC with DMAEMA (CMC-g-PDMAEMA) | 118–133 (nanocapsule) | Drug delivery (anticancer drug: paclitaxel) | [198] |
| HEC | Methodology: graft polymerization of CEHEC with adamantane (CEHEC-g-Ad) followed by self-assembly with GE-CD and CD-DOX | 36.4 ± 2.2 (nanocapsule) | Drug delivery (anticancer drug: DOX) | [199] |
| HPMC | Methodology: graft polymerization of HPMC with PLA (HPMC-g-PLA) | 175–216 (nanocapsule) | Drug delivery (anticancer drug: paclitaxel) | [200] |
| Lyocell (TENCEL™ Lyocell) | Methodology: carboxymethylation of TENCEL™ gel followed by homogenization in a high-pressure homogenizer | 73–129 (nanosphere) | – | [203] |
| Lyocell (LENZING™ Lyocell) | Methodology: carboxymethylation of lyocell fibres followed by homogenization in a microfluidizer | 16 ± 5 (TEM) 22 ± 7 (AFM) 51 ± 4 (DLS) (nanocapsule) | – | [204] |
| Lyocell (LENZING™ Lyocell) | Methodology: heterogenous modification of lyocell gel with glycidyltrimethylammonium chloride followed by mechanical shearing in a microfluidizer | 30 ± 8 (AFM) 55 ± 8 (DLS) (nanocapsule) | Immunoassays (proteins: hIgG, BSA) | [205] |
| LAYER-BY-LAYER ASSEMBLY | ||||
| CMC | Methodology: assembly of 3 CMC/protamine bilayers on a silica sacrificial template, followed by surface decoration with ferrite nanoparticles | 150 ± 20 (nanocapsule) | Drug delivery (anticancer drug: DOX) | [183] |
| CMC and QHECE | Methodology: LbL deposition of CMC and QHECE on a cationic vesicular template of DDAB | 306 (1st layer) up to 1,600 (6th layer) (nanocapsules) | Potential for drug delivery | [206] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. https://doi.org/10.3390/nano11102744
Carvalho JPF, Silva ACQ, Silvestre AJD, Freire CSR, Vilela C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials. 2021; 11(10):2744. https://doi.org/10.3390/nano11102744
Chicago/Turabian StyleCarvalho, João P. F., Ana C. Q. Silva, Armando J. D. Silvestre, Carmen S. R. Freire, and Carla Vilela. 2021. "Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications" Nanomaterials 11, no. 10: 2744. https://doi.org/10.3390/nano11102744
APA StyleCarvalho, J. P. F., Silva, A. C. Q., Silvestre, A. J. D., Freire, C. S. R., & Vilela, C. (2021). Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials, 11(10), 2744. https://doi.org/10.3390/nano11102744