Cyanogel-Derived Synthesis of Porous PdFe Nanohydrangeas as Electrocatalysts for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of PdFe Porous Nanohydrangeas
2.3. Characterizations
2.4. Electrochemical Measurements
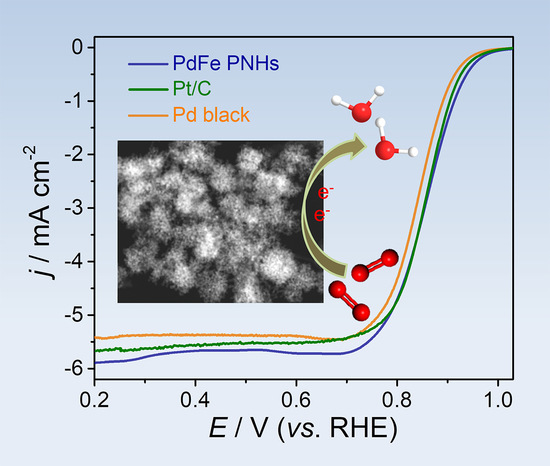
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.T.; Liu, X.F.; Zheng, L.R.; Shui, J.L. The Solid-Phase Synthesis of an Fe-N-C Electrocatalyst for High-Power Proton-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2018, 57, 1204–1208. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Shao, Z.G.; Zhang, H.J.; Wang, Z.Q.; Hong, S.J.; Yu, H.M.; Yi, B.L. Nanostructured ultrathin catalyst layer based on open-walled PtCo bimetallic nanotube arrays for proton exchange membrane fuel cells. Nano Energy 2017, 34, 344–355. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Y.; Yang, Y.; Tang, X.; Peng, Y.Q.; Peng, H.Q.; Xiao, L.; Lu, J.T.; Abruña, H.D.; Zhuang, L. Fe/N/C Nanotubes with Atomic Fe Sites: A Highly Active Cathode Catalyst for Alkaline Polymer Electrolyte Fuel Cells. ACS Catal. 2017, 7, 6485–6492. [Google Scholar] [CrossRef]
- Liu, H.P.; Zhong, P.; Liu, K.; Han, L.; Zheng, H.Q.; Yin, Y.D.; Gao, C.B. Synthesis of ultrathin platinum nanoplates for enhanced oxygen reduction activity. Chem. Sci. 2018, 9, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.Y.; Liang, X.; Xu, G.C.; Li, D.W.; Li, Y.; Zhang, N.; Chen, Y.Z.; Jiang, X.F.; Gong, H.Y. Self-Supported Defect-Rich Au-Based Nanostructures as Robust Bifunctional Catalysts for the Methanol Oxidation Reaction and Oxygen Reduction Reaction in an Alkaline Medium. Nanomaterials 2021, 11, 2193. [Google Scholar] [CrossRef]
- Chen, W.C.; Yang, G.; Zhao, Y.; Yuan, G.Q.; Ye, J.S.; Liu, H.Y.; Xiao, X.Y. Porous carbon polyhedrons with exclusive Metal-Nx moieties for efficient oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 39882–39891. [Google Scholar] [CrossRef]
- He, B.H.; Chen, Y.Y.; Hu, D.; Wen, Z.Y.; Zhou, M.J.; Xu, W.Y. N-Doped hierarchical porous carbon nanoscrolls towards efficient oxygen reduction reaction in Zn-air batteries via interior and exterior modifications. Mater. Adv. 2021, 2, 7036–7044. [Google Scholar] [CrossRef]
- Falco, G.; Florent, M.; Jagiello, J.; Cheng, Y.Q.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Bandosz, T.J. Alternative view of oxygen reduction on porous carbon electrocatalysts: The substance of complex oxygen-surface interactions. Iscience 2021, 24, 102216. [Google Scholar] [CrossRef]
- Han, M.N.; Shi, M.J.; Wang, J.; Zhang, M.L.; Yan, C.; Jiang, J.T.; Guo, S.H.; Sun, Z.Y.; Guo, Z.H. Efficient bifunctional Co/N dual-doped carbon electrocatalysts for oxygen reduction and evolution reaction. Carbon 2019, 153, 575–584. [Google Scholar] [CrossRef]
- Li, Z.S.; Li, Y.Y.; He, C.Y.; Shen, P.K. Bimetallic PtAg alloyed nanoparticles and 3-D mesoporous graphene nanosheets hybrid architectures for advanced oxygen reduction reaction electrocatalysts. J. Mater. Chem. A 2017, 5, 23158–23169. [Google Scholar] [CrossRef]
- Jiang, K.Z.; Zhao, D.D.; Guo, S.J.; Zhang, X.; Zhu, X.; Guo, J.; Lu, G.; Huang, X.Q. Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires. Sci. Adv. 2017, 3, e1601705. [Google Scholar] [CrossRef] [Green Version]
- Pavlets, A.; Alekseenko, A.; Menshchikov, V.; Belenov, S.; Volochaev, V.; Pankov, I.; Safronenko, O.; Guterman, V. Influence of Electrochemical Pretreatment Conditions of PtCu/C Alloy Electrocatalyst on Its Activity. Nanomaterials 2021, 11, 1499. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, X.Y.; Cui, L.R.; Shi, Z.P.; Lu, B.Q.; Guo, X.M.; Zhang, J.B.; Xu, L.; Tang, Y.W.; Xiang, Y. High-Performance Oxygen Reduction Electrocatalysis Enabled by 3D PdNi Nanocorals with Hierarchical Porosity. Part. Part. Syst. Charact. 2018, 35, 1700366. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Fu, G.T.; Li, J.H.; Liu, Z.Q.; Xu, L.; Sun, D.M.; Tang, Y.W. Facile synthesis based on novel carbon-supported cyanogel of structurally ordered Pd3Fe/C as electrocatalyst for formic acid oxidation. Nano Res. 2018, 11, 4686–4696. [Google Scholar] [CrossRef]
- Elsheikh, A.; McGregor, J. Synthesis and Characterization of PdAgNi/C Trimetallic Nanoparticles for Ethanol Electrooxidation. Nanomaterials 2021, 11, 2244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Jiang, J.T.; Yuan, Y.Y.; Jiang, Q.L.; Yan, C. Vertically Aligned NiCo2O4 Nanosheet-Encapsulated Carbon Fibers as a Self-Supported Electrode for Superior Li+ Storage Performance. Nanomaterials 2019, 9, 1336. [Google Scholar] [CrossRef] [Green Version]
- Goswami, C.; Saikia, H.; Tada, K.; Tanaka, S.; Sudarsanam, P.; Bhargava, S.K.; Bharali, P. Bimetallic Palladium–Nickel Nanoparticles Anchored on Carbon as High-Performance Electrocatalysts for Oxygen Reduction and Formic Acid Oxidation Reactions. ACS Appl. Energy Mater. 2020, 3, 9285–9295. [Google Scholar] [CrossRef]
- Wu, D.F.; Cheng, D.J. Structure-controlled synthesis of one-dimensional PdCu nanoscatalysts via a seed-mediated approach for oxygen reduction reaction. Appl. Surf. Sci. 2019, 493, 139–145. [Google Scholar] [CrossRef]
- Li, X.; Li, X.X.; Liu, C.X.; Huang, H.W.; Gao, P.F.; Ahmad, F.; Luo, L.H.; Ye, Y.F.; Geng, Z.G.; Wang, G.X.; et al. Atomic-Level Construction of Tensile-Strained PdFe Alloy Surface toward Highly Efficient Oxygen Reduction Electrocatalysis. Nano Lett. 2020, 20, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Choi, D.; Cho, J.; Park, H.Y.; Lee, K.S.; Ahn, M.; Jang, I.; Park, T.; Ham, H.C.; Yoo, S.J. Highly Active and Durable Ordered Intermetallic PdFe Electrocatalyst for Formic Acid Electrooxidation Reaction. ACS Appl. Energy Mater. 2020, 3, 4226–4237. [Google Scholar] [CrossRef]
- Yang, S.W.; Chung, Y.J.; Lee, K.S.; Kwon, Y.C. Enhancements in catalytic activity and duration of PdFe bimetallic catalysts and their use in direct formic acid fuel cells. J. Ind. Eng. Chem. 2020, 90, 351–357. [Google Scholar] [CrossRef]
- Douk, A.S.; Farsadrooh, M.; Damanigol, F.; Moghaddam, A.A.; Saravani, H.; Noroozifar, M. Porous three-dimensional network of Pd–Cu aerogel toward formic acid oxidation. RSC Adv. 2018, 8, 23539–23545. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Shang, H.Y.; Wang, C.; Jin, L.J.; Chen, C.Y.; Du, Y.K. Nanoscale engineering of porous Fe-doped Pd nanosheet assemblies for efficient methanol and ethanol electrocatalyses. Nanoscale 2020, 12, 2126–2132. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xie, C.; Liu, D.D.; Huang, X.B.; Huo, J.; Wang, S.Y. Nanoparticles-Stacked Porous Nickel-Iron Nitride Nanosheet: A Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 18652–18657. [Google Scholar] [CrossRef]
- Qin, Q.; Xie, J.; Dong, Q.Z.; Yu, G.; Chen, H. Surfactant-free fabrication of porous PdSn alloy networks by self-assembly as superior freestanding electrocatalysts for formic acid oxidation. N. J. Chem. 2019, 43, 19242–19252. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Gerbasi, R.; Guerriero, P.; Musiani, M.; Verlato, E. Electrodeposition of compact and porous Cu-Pd alloy layers and their application to nitrate reduction in alkali. Electrochim. Acta 2017, 230, 365–372. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Gerbasi, R.; Guerriero, P.; Musiani, M.; Vázquez-Gómez, L. Preparation of compact and porous Pd-Ni alloys and study of their performances for ethanol oxidation in alkali. Electrochim. Acta 2019, 307, 503–511. [Google Scholar] [CrossRef]
- Sheng, J.L.; Kang, J.H.; Ye, H.Q.; Xie, J.Q.; Zhao, B.; Fu, X.Z.; Yu, Y.; Sun, R.; Wong, C.P. Porous octahedral PdCu nanocages as highly efficient electrocatalysts for the methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 3906–3912. [Google Scholar] [CrossRef]
- Guo, J.C.; Gao, L.; Tan, X.; Yuan, Y.L.; Kim, J.; Wang, Y.; Wang, H.; Zeng, Y.J.; Choi, S.I.; Smith, S.C.; et al. Template-Directed Rapid Synthesis of Pd-Based Ultrathin Porous Intermetallic Nanosheets for Efficient Oxygen Reduction. Angew. Chem. Int. Ed. 2021, 60, 10942–10949. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, B.W.; Bocarsly, A.B.; Prud’homme, R.K. Synthesis of a novel hydrogel based on a coordinate covalent polymer network. J. Am. Chem. Soc. 1993, 115, 2661–2665. [Google Scholar] [CrossRef]
- Heibel, M.; Kumar, G.; Wyse, C.; Bukovec, P.; Bocarsly, A.B. Use of sol-gel chemistry for the preparation of cyanogels as ceramic and alloy precursors. Chem. Mater. 1996, 8, 1504–1511. [Google Scholar] [CrossRef]
- Vondrova, M.; McQueen, T.M.; Burgess, C.M.; Ho, D.M.; Bocarsly, A.B. Autoreduction of Pd−Co and Pt−Co cyanogels: Exploration of cyanometalate coordination chemistry at elevated temperatures. J. Am. Chem. Soc. 2008, 130, 5563–5572. [Google Scholar] [CrossRef]
- Peng, X.W.; Ye, W.; Ding, Y.C.; Jiang, S.H.; Hanif, M.; Liao, X.J.; Hou, H.Q. Facile synthesis, characterization and application of highly active palladium nano-network structures supported on electrospun carbon nanofibers. RSC Adv. 2014, 4, 42732–42736. [Google Scholar] [CrossRef]
- Su, Y.H.; Jiang, H.L.; Zhu, Y.H.; Yang, X.L.; Shen, J.H.; Zou, W.J.; Chen, J.D.; Li, C.Z. Enriched graphitic N-doped carbon-supported Fe3O4 nanoparticles as efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7281–7287. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Xu, A.W. Noble-Metal-Free Fe–N/C Catalyst for Highly Efficient Oxygen Reduction Reaction under Both Alkaline and Acidic Conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef]
- Kong, A.G.; Zhu, X.F.; Han, Z.; Yu, Y.Y.; Zhang, Y.B.; Dong, B.; Shan, Y.K. Ordered Hierarchically Micro- and Mesoporous Fe–Nx-Embedded Graphitic Architectures as Efficient Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2014, 4, 1793–1800. [Google Scholar] [CrossRef]
- Hu, S.; Munoz, F.; Noborikawa, J.; Haan, J.; Scudiero, L.; Ha, S. Carbon supported Pd-based bimetallic and trimetallic catalyst for formic acid electrochemical oxidation. Appl. Catal. B Environ. 2016, 180, 758–765. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, L.; Ma, Y.R.; Chen, Y.; Zhou, Y.M.; Tang, Y.W.; Lu, T.H. Crystalline palladium–cobalt alloy nanoassemblies with enhanced activity and stability for the formic acid oxidation reaction. Appl. Catal. B Environ. 2013, 138, 229–235. [Google Scholar] [CrossRef]
- Xu, C.X.; Liu, Y.Q.; Hao, Q.; Duan, H.M. Nanoporous PdNi alloys as highly active and methanol-tolerant electrocatalysts towards oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 13542–13548. [Google Scholar] [CrossRef]
- Jiang, G.M.; Zhu, H.Y.; Zhang, X.; Shen, B.; Wu, L.H.; Zhang, S.; Lu, G.; Wu, Z.B.; Sun, S.H. Core/Shell Face-Centered Tetragonal FePd/Pd Nanoparticles as an Efficient Non-Pt Catalyst for the Oxygen Reduction Reaction. ACS Nano 2015, 9, 11014–11022. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Mu, X.Q.; Duan, H.Y.; Chen, C.Y.; Zhang, H. Pd Nanoparticle Assemblies as Efficient Catalysts for the Hydrogen Evolution and Oxygen Reduction Reactions. Eur. J. Inorg. Chem. 2017, 2017, 535–539. [Google Scholar] [CrossRef]
- Zhong, X.; Qin, Y.Y.; Chen, X.L.; Xu, W.L.; Zhuang, G.; Li, X.N.; Wang, J.G. PtPd alloy embedded in nitrogen-rich graphene nanopores: High-performance bifunctional electrocatalysts for hydrogen evolution and oxygen reduction. Carbon 2017, 114, 740–748. [Google Scholar] [CrossRef]
- Goswami, C.; Saikia, H.; Jyoti Borah, B.; Jyoti Kalita, M.; Tada, K.; Tanaka, S.; Bharali, P. Boosting the electrocatalytic activity of Pd/C by Cu alloying: Insight on Pd/Cu composition and reaction pathway. J. Colloid Interface Sci. 2021, 587, 446–456. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, K.; Tang, Z.H.; Liu, Z.; Chen, S.W. Bimetallic PdZn nanoparticles for oxygen reduction reaction in alkaline medium: The effects of surface structure. J. Catal. 2020, 382, 181–191. [Google Scholar] [CrossRef]
- Thi, M.L.N.; Tran, T.H.; Anh, P.D.H.; Nhac-Vu, H.T.; Bui, Q.B. An innovative catalyst of nickel-palladium alloy nanocrystals embedded nitrogen-doped graphene for efficient oxygen reduction reaction. J. Alloys Compd 2019, 797, 314–324. [Google Scholar] [CrossRef]
- Lu, Y.N.; Zhao, S.L.; Yang, R.; Xu, D.D.; Yang, J.; Lin, Y.; Shi, N.E.; Dai, Z.H.; Bao, J.C.; Han, M. Well-Coupled Nanohybrids Obtained by Component-Controlled Synthesis and in Situ Integration of MnxPdy Nanocrystals on Vulcan Carbon for Electrocatalytic Oxygen Reduction. ACS Appl. Mater. Interfaces 2018, 10, 8155–8164. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Zhang, Q.H.; Li, Y.F.; Han, M.; Gu, L.; Nan, C.W.; Bao, J.C.; Dai, Z.H. Five-Fold Twinned Pd2NiAg Nanocrystals with Increased Surface Ni Site Availability to Improve Oxygen Reduction Activity. J. Am. Chem. Soc. 2015, 137, 2820–2823. [Google Scholar] [CrossRef]
- Xue, H.R.; Tang, J.; Gong, H.; Guo, H.; Fan, X.L.; Wang, T.; He, J.P.; Yamauchi, Y. Fabrication of PdCo Bimetallic Nanoparticles Anchored on Three-Dimensional Ordered N-Doped Porous Carbon as an Efficient Catalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 20766–20771. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Liu, Z.; Yang, X.; Cheng, P.; Yan, C. Cyanogel-Derived Synthesis of Porous PdFe Nanohydrangeas as Electrocatalysts for Oxygen Reduction Reaction. Nanomaterials 2021, 11, 3382. https://doi.org/10.3390/nano11123382
Wan J, Liu Z, Yang X, Cheng P, Yan C. Cyanogel-Derived Synthesis of Porous PdFe Nanohydrangeas as Electrocatalysts for Oxygen Reduction Reaction. Nanomaterials. 2021; 11(12):3382. https://doi.org/10.3390/nano11123382
Chicago/Turabian StyleWan, Jinxin, Zhenyuan Liu, Xiaoyu Yang, Peng Cheng, and Chao Yan. 2021. "Cyanogel-Derived Synthesis of Porous PdFe Nanohydrangeas as Electrocatalysts for Oxygen Reduction Reaction" Nanomaterials 11, no. 12: 3382. https://doi.org/10.3390/nano11123382
APA StyleWan, J., Liu, Z., Yang, X., Cheng, P., & Yan, C. (2021). Cyanogel-Derived Synthesis of Porous PdFe Nanohydrangeas as Electrocatalysts for Oxygen Reduction Reaction. Nanomaterials, 11(12), 3382. https://doi.org/10.3390/nano11123382