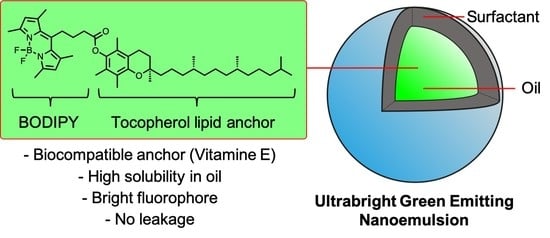
Ultrabright Green-Emitting Nanoemulsions Based on Natural Lipids-BODIPY Conjugates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Spectroscopy
2.3. Solubility Test in Oils
2.4. Formulation and Characterization of Nanoemulsions
2.5. Characterization and Stability of Nanoemulsions
2.6. Photostability Test
2.7. Fluorescence Correlation Spectroscopy (FCS)
3. Results
3.1. Synthesis of the Lipid-BODIPY Conjugates
3.2. Photophysical Studies of BODIPY Conjugates
3.3. Solubility of BDP Conjugates in Oils
3.4. BDP-Loaded Nanoemulsions
3.5. Dye Leakage
3.6. Photostability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Mudassir, J.; Mohtar, N.; Darwis, Y. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013, 8, 2733. [Google Scholar]
- Anton, N.; Vandamme, T.F. The universality of low-energy nano-emulsification. Int. J. Pharm 2009, 377, 142–147. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Yao, M.; Xiao, H.; McClements, D.J. Delivery of lipophilic bioactives: Assembly, disassembly, and reassembly of lipid nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Bou, S.; Wang, X.; Anton, N.; Klymchenko, A.S.; Collot, M. Near infrared fluorogenic probe as a prodrug model for evaluating cargo release by nanoemulsions. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Bouchaala, R.; Mercier, L.; Andreiuk, B.; Mély, Y.; Vandamme, T.; Anton, N.; Goetz, J.G.; Klymchenko, A.S. Integrity of lipid nanocarriers in bloodstream and tumor quantified by near-infrared ratiometric FRET imaging in living mice. J. Control. Release 2016, 236, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Anton, N.; Ashokkumar, P.; Anton, H.; Fam, T.K.; Vandamme, T.; Klymchenko, A.S.; Collot, M. Optimizing the Fluorescence Properties of Nanoemulsions for Single Particle Tracking in Live Cells. ACS Appl. Mater. Interfaces 2019, 11, 13079–13090. [Google Scholar] [CrossRef] [Green Version]
- Klymchenko, A.S.; Liu, F.; Collot, M.; Anton, N. Dye-Loaded Nanoemulsions: Biomimetic Fluorescent Nanocarriers for Bioimaging and Nanomedicine. Adv. Healthc. Mater. 2021, 10, 2001289. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Roger, E.; Anton, N.; Anton, H.; Shulov, I.; Vermot, J.; Mely, Y.; Vandamme, T.F. Highly lipophilic fluorescent dyes in nano-emulsions: Towards bright non-leaking nano-droplets. RSC Adv. 2012, 2, 11876–11886. [Google Scholar] [CrossRef] [Green Version]
- Kilin, V.N.; Anton, H.; Anton, N.; Steed, E.; Vermot, J.; Vandamme, T.F.; Mely, Y.; Klymchenko, A.S. Counterion-enhanced cyanine dye loading into lipid nano-droplets for single-particle tracking in zebrafish. Biomaterials 2014, 35, 4950–4957. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gaytan, B.L.; Fay, F.; Hak, S.; Alaarg, A.; Fayad, Z.A.; Pérez-Medina, C.; Mulder, W.J.M.; Zhao, Y. Real-Time Monitoring of Nanoparticle Formation by FRET Imaging. Angew. Chem. Int. Ed. 2017, 56, 2923–2926. [Google Scholar] [CrossRef] [Green Version]
- An, H.Z.; Helgeson, M.E.; Doyle, P.S. Nanoemulsion Composite Microgels for Orthogonal Encapsulation and Release. Adv. Mater. 2012, 24, 3838–3844. [Google Scholar] [CrossRef]
- Saito, A.; Yamamoto, S.; Ochi, R.; Inoue, K.; Hadano, S.; Watanabe, S.; Nakayama, T.; Niko, Y. An Azide-Tethered Cremophor® ELP Surfactant Allowing Facile Post-Surface Functionalization of Nanoemulsions. Bull. Chem. Soc. Jpn. 2020, 93, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Collot, M.; Boutant, E.; Lehmann, M.; Klymchenko, A.S. BODIPY with Tuned Amphiphilicity as a Fluorogenic Plasma Membrane Probe. Bioconjugate Chem. 2019, 30, 192–199. [Google Scholar] [CrossRef]
- Thomsen, T.; Ayoub, A.B.; Psaltis, D.; Klok, H.-A. Fluorescence-Based and Fluorescent Label-Free Characterization of Polymer Nanoparticle Decorated T Cells. Biomacromolecules 2021, 22, 190–200. [Google Scholar] [CrossRef]
- Operti, M.C.; Dölen, Y.; Keulen, J.; van Dinther, E.A.W.; Figdor, C.G.; Tagit, O. Microfluidics-Assisted Size Tuning and Biological Evaluation of PLGA Particles. Pharmaceutics 2019, 11, 590. [Google Scholar] [CrossRef] [Green Version]
- Trofymchuk, K.; Valanciunaite, J.; Andreiuk, B.; Reisch, A.; Collot, M.; Klymchenko, A.S. BODIPY-loaded polymer nanoparticles: Chemical structure of cargo defines leakage from nanocarrier in living cells. J. Mater. Chem. B 2019, 7, 5199–5210. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hong, X.; Zong, S.; Tang, C.; Cui, Y.; Zheng, Q. BODIPY-doped silica nanoparticles with reduced dye leakage and enhanced singlet oxygen generation. Sci. Rep. 2015, 5, 12602. [Google Scholar] [CrossRef] [Green Version]
- Bou, S.; Wang, X.; Anton, N.; Bouchaala, R.; Klymchenko, A.S.; Collot, M. Lipid-core/polymer-shell hybrid nanoparticles: Synthesis and characterization by fluorescence labeling and electrophoresis. Soft Matter 2020, 16, 4173–4181. [Google Scholar] [CrossRef]
- Noweck, K.; Grafahrend, W. Fatty Alcohols. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: Atlanta, GA, USA, 2006; ISBN 978-3-527-30673-2. [Google Scholar]
- Principles of Fluorescence Spectroscopy; Lakowicz, J.R. (Ed.) Springer US: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Attia, M.F.; Dieng, S.M.; Collot, M.; Klymchenko, A.S.; Bouillot, C.; Serra, C.A.; Schmutz, M.; Er-Rafik, M.; Vandamme, T.F.; Anton, N. Functionalizing Nanoemulsions with Carboxylates: Impact on the Biodistribution and Pharmacokinetics in Mice. Macromol. Biosci. 2017, 17, 1600471. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Schwille, P.; Weidemann, T. PyCorrFit—generic data evaluation for fluorescence correlation spectroscopy. Bioinformatics 2014, 30, 2532–2533. [Google Scholar] [CrossRef] [Green Version]
- BODIPYTM FL C12-Sphingomyelin (N-(4,4-Difluoro-5,7-Dimethyl-4-Bora-3a,4a-Diaza-s-Indacene-3-Dodecanoyl) Sphingosyl Phosphocholine). Available online: https://www.thermofisher.com/order/catalog/product/D7711 (accessed on 21 January 2021).
- Life Technologies, Thermo Fisher Scientific.
- Li, Z.; Mintzer, E.; Bittman, R. First Synthesis of Free Cholesterol−BODIPY Conjugates. J. Org. Chem. 2006, 71, 1718–1721. [Google Scholar] [CrossRef]
- Wang, D.; Fan, J.; Gao, X.; Wang, B.; Sun, S.; Peng, X. Carboxyl BODIPY Dyes from Bicarboxylic Anhydrides: One-Pot Preparation, Spectral Properties, Photostability, and Biolabeling. J. Org. Chem. 2009, 74, 7675–7683. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Chiper, M.; Akasov, R.; Anton, H.; Messaddeq, N.; Fournel, S.; Klymchenko, A.S.; Mély, Y.; Vandamme, T.F. Biodistribution of X-Ray Iodinated Contrast Agent in Nano-Emulsions Is Controlled by the Chemical Nature of the Oily Core. ACS Nano 2014, 8, 10537–10550. [Google Scholar] [CrossRef]
- Collot, M.; Schild, J.; Fam, K.T.; Bouchaala, R.; Klymchenko, A.S. Stealth and Bright Monomolecular Fluorescent Organic Nanoparticles Based on Folded Amphiphilic Polymer. ACS Nano 2020. [Google Scholar] [CrossRef]
- Wang, X.; Collot, M.; Omran, Z.; Vandamme, T.F.; Klymchenko, A.; Anton, N. Further insights into release mechanisms from nano-emulsions, assessed by a simple fluorescence-based method. J. Colloid Interface Sci. 2020, 578, 768–778. [Google Scholar] [CrossRef]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Wöll, D. Fluorescence correlation spectroscopy in polymer science. RSC Adv. 2013, 4, 2447–2465. [Google Scholar] [CrossRef]
- Jurkiewicz, B.A.; Bissett, D.L.; Buettner, G.R. Effect of Topically Applied Tocopherol on Ultraviolet Radiation-Mediated Free Radical Damage in Skin. J. Investig. Dermatol. 1995, 104, 484–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vaugelade, S.; Nicol, E.; Vujovic, S.; Bourcier, S.; Pirnay, S.; Bouchonnet, S. UV–vis degradation of α-tocopherol in a model system and in a cosmetic emulsion—Structural elucidation of photoproducts and toxicological consequences. J. Chromatogr. A 2017, 1517, 126–133. [Google Scholar] [CrossRef] [PubMed]






| BDP-Toco | ||||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | λAbs (nm) | ε (M−1 cm−1) | FWHM Abs (nm) | λEm (nm) | FWHM Em (nm) | Stokes Shift (nm) | φ a (QY) | Brightness b (M−1 cm−1) |
| VEA | 501 | 74,800 | 21 | 508 | 24 | 7 | 0.98 | 73,400 |
| MCT | 501 | 82,400 | 18 | 508 | 24 | 7 | 0.92 | 75,600 |
| Cyclohexane | 502 | 90,900 | 20 | 508 | 28 | 6 | 0.88 | 80,100 |
| Dioxane | 500 | 83,100 | 18 | 507 | 26 | 7 | 0.83 | 68,800 |
| ACN | 496 | 75,100 | 19 | 504 | 27 | 8 | 0.75 | 56,700 |
| MeOH | 498 | 82,900 | 19 | 505 | 29 | 7 | 0.78 | 64,400 |
| Water | 507 | 32,800 | 59 | 699 | N/A c | N/A c | 0.04 | 1250 |
| BDP-Chol | ||||||||
| Solvent | λAbs(nm) | ε (M−1 cm−1) | FWHM Abs (nm) | λEm(nm) | FWHM Em (nm) | Stokes Shift (nm) | φ a (QY) | Brightnessb (M−1 cm−1) |
| VEA | 501 | 70,000 | 21 | 507 | 24 | 6 | 0.89 | 62,000 |
| MCT | 501 | 73,500 | 18 | 507 | 24 | 6 | 0.92 | 67,700 |
| Cyclohexane | 502 | 77,000 | 16 | 508 | 28 | 6 | 0.81 | 62,700 |
| Dioxane | 500 | 75,100 | 18 | 507 | 24 | 7 | 0.76 | 56,900 |
| ACN | 496 | 68,900 | 19 | 504 | 28 | 8 | 0.67 | 46,500 |
| MeOH | 497 | 70,800 | 16 | 505 | 29 | 8 | 0.73 | 51,800 |
| Water | 507 | 33,800 | 56 | 570 | N/A c | N/A c | 0.18 | 6000 |
| BDP-C18 | ||||||||
| Solvent | λAbs(nm) | ε (M−1 cm−1) | FWHM Abs (nm) | λEm(nm) | FWHM Em (nm) | Stokes shift (nm) | φ a (QY) | Brightnessb (M−1 cm−1) |
| VEA | 501 | 64,600 | 21 | 508 | 24 | 7 | 0.99 | 64,100 |
| MCT | 501 | 73,900 | 18 | 507 | 25 | 6 | 0.95 | 70,400 |
| Cyclohexane | 502 | 81,200 | 16 | 508 | 29 | 6 | 0.82 | 66,700 |
| Dioxane | 499 | 74,400 | 18 | 507 | 27 | 8 | 0.8 | 59,100 |
| ACN | 496 | 66,800 | 19 | 504 | 28 | 8 | 0.68 | 45,600 |
| MeOH | 497 | 68,900 | 19 | 505 | 29 | 8 | 0.74 | 50,700 |
| Water | 503 | 28,600 | 57 | 576 | N/A c | N/A c | 0.13 | 3800 |
| Oil | BDP-Toco | BDP-Chol | BDP-C18 |
|---|---|---|---|
| MCT | >12 wt % | 1.2 wt % | 2.9 wt % |
| VEA | >12 wt % | 3.3 wt % | 2.7 wt % |
| BDP | Oil | Medium | Size (nm) | Concentration (nM) | BDP | Oil | Medium | Size (nm) | Concentration (nM) | Concentration Ratio (FBS/PBS) |
|---|---|---|---|---|---|---|---|---|---|---|
| BDP-Toco | MCT | PBS | 64 | 5.84 | BDP-Toco | MCT | FBS | 62 | 4.79 | 0.82 |
| BDP-Chol | MCT | PBS | 86 | 2.83 | BDP-Chol | MCT | FBS | 84 | 2.97 | 1.05 |
| BDP-C18 | MCT | PBS | 79 | 1.83 | BDP-C18 | MCT | FBS | 78 | 2.13 | 1.16 |
| BDP-Toco | VEA | PBS | 45 | 7.63 | BDP-Toco | VEA | FBS | 47 | 9.32 | 1.22 |
| BDP-Chol | VEA | PBS | 50 | 8.18 | BDP-Chol | VEA | FBS | 51 | 9.08 | 1.11 |
| BDP-C18 | VEA | PBS | 51 | 5.73 | BDP-C18 | VEA | FBS | 53 | 6.93 | 1.21 |
| BDP-Toco Loading | Oil | Medium | Size (nm) | Concentration (nM) | Concentration Ratio (FBS/PBS) |
|---|---|---|---|---|---|
| 1 wt % | MCT | PBS/FBS | 64/62 | 5.84/4.79 | 0.82 |
| 3 wt % | MCT | PBS/FBS | 60/63 | 1.48/1.68 | 1.14 |
| 6 wt % | MCT | PBS/FBS | 56/61 | 0.75/1.35 | 1.80 |
| 10 wt % | MCT | PBS/FBS | 64/74 | 0.46/0.97 | 2.11 |
| 12 wt % | MCT | PBS/FBS | 59/73 | 0.47/0.85 | 1.81 |
| BDP-Toco Loading | Oil | Medium | Size (nm) | Concentration (nM) | Concentration Ratio (FBS/PBS) |
| 1 wt % | VEA | PBS/FBS | 45/47 | 7.63/9.32 | 1.22 |
| 3 wt % | VEA | PBS/FBS | 46/47 | 2.43/3.55 | 1.46 |
| 6 wt % | VEA | PBS/FBS | 42/43 | 1.32/2.93 | 2.22 |
| 10 wt % | VEA | PBS/FBS | 47/46 | 1.27/2.36 | 1.86 |
| 12 wt % | VEA | PBS/FBS | 52/53 | 1.05/1.77 | 1.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Bou, S.; Klymchenko, A.S.; Anton, N.; Collot, M. Ultrabright Green-Emitting Nanoemulsions Based on Natural Lipids-BODIPY Conjugates. Nanomaterials 2021, 11, 826. https://doi.org/10.3390/nano11030826
Wang X, Bou S, Klymchenko AS, Anton N, Collot M. Ultrabright Green-Emitting Nanoemulsions Based on Natural Lipids-BODIPY Conjugates. Nanomaterials. 2021; 11(3):826. https://doi.org/10.3390/nano11030826
Chicago/Turabian StyleWang, Xinyue, Sophie Bou, Andrey S. Klymchenko, Nicolas Anton, and Mayeul Collot. 2021. "Ultrabright Green-Emitting Nanoemulsions Based on Natural Lipids-BODIPY Conjugates" Nanomaterials 11, no. 3: 826. https://doi.org/10.3390/nano11030826
APA StyleWang, X., Bou, S., Klymchenko, A. S., Anton, N., & Collot, M. (2021). Ultrabright Green-Emitting Nanoemulsions Based on Natural Lipids-BODIPY Conjugates. Nanomaterials, 11(3), 826. https://doi.org/10.3390/nano11030826