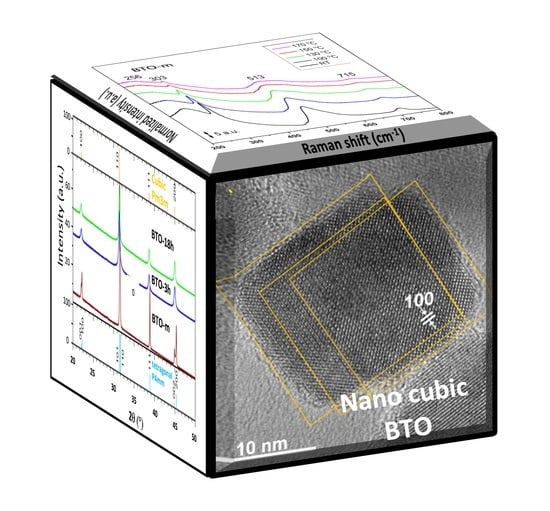
Structural Details of BaTiO3 Nano-Powders Deduced from the Anisotropic XRD Peak Broadening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BTO Preparation Procedure
2.3. Characterization of the BTO Samples
3. Results and Discussion
3.1. TEM Investigations
3.2. XRD Analysis
3.2.1. Isotropic Approximation
The Structure at Room Temperature
The Structure above Room Temperature
3.2.2. The hkl-Dependent XRD Peak Broadening: Evidence of Tetragonal Deformation, Cubic Shape and Surface Relaxation
3.3. Raman Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mimura, K. Processing of dielectric nanocube 3D-assemblies and their high electrical properties for next-generation devices. J. Ceram. Soc. Jpn. 2016, 124, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Hoshina, T. Size effect of barium titanate: Fine particles and ceramics. J. Ceram. Soc. Jpn. 2013, 121, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Tsurumi, T.; Sekine, T.; Kakemoto, H.; Hoshina, T.; Nam, S.M.; Yasuno, H.; Wada, S. Evaluation and Statistical Analysis of Dielectric Permittivity of BaTiO3 Powders. J. Am. Ceram. Soc. 2006, 89, 1337–1341. [Google Scholar] [CrossRef]
- Polking, M.J.; Han, M.-G.; Yourdkhani, A.; Petkov, V.; Kisielowski, C.F.; Volkov, V.V.; Zhu, Y.; Caruntu, G.; Alivisatos, A.P.; Ramesh, R. Ferroelectric order in individual nanometre-scale crystals. Nat. Mater. 2012, 11, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Mimura, K.; Kato, K.; Imai, H.; Wada, S.; Haneda, H.; Kuwabara, M. Piezoresponse properties of orderly assemblies of BaTiO3 and SrTiO3 nanocube single crystals. Appl. Phys. Lett. 2012, 101, 012901. [Google Scholar] [CrossRef] [Green Version]
- Mimura, K.; Kato, K. Dielectric properties of barium zirconate titanate nanocube 3D-ordered assemblies. J. Ceram. Soc. Jpn. 2018, 126, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Walton, R.I. Perovskite Oxides Prepared by Hydrothermal and Solvothermal Synthesis: A Review of Crystallisation, Chemistry, and Compositions. Chem. Eur. J. 2020, 26, 9041–9069. [Google Scholar] [CrossRef] [PubMed]
- Joshi, U.A.; Lee, J.S. Template-free hydrothermal synthesis of single-crystalline barium titanate and strontium titanate nanowires. Small 2005, 1, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Brus, L.; Murray, C.B. Synthesis of Monodisperse Nanoparticles of Barium Titanate: Toward a Generalized Strategy of Oxide Nanoparticle Synthesis. J. Am. Chem. Soc. 2001, 123, 12085–12086. [Google Scholar] [CrossRef]
- Jiang, B.; Pang, X.; Li, B.; Lin, Z. Organic–Inorganic Nanocomposites via Placing Monodisperse Ferroelectric Nanocrystals in Direct and Permanent Contact with Ferroelectric Polymers. J. Am. Chem. Soc. 2015, 137, 11760–11767. [Google Scholar] [CrossRef]
- Deng, Z.; Dai, Y.; Chen, W.; Pei, X.M.; Liao, J.H. Synthesis and Characterization of Bowl-Like Single-Crystalline BaTiO3 Nanoparticles. Nanoscale Res. Lett. 2010, 5, 1217–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, N.Z.; Shen, L.M.; Gupta, A.; Tatarenko, A.; Srinivasan, G.; Yanagisawa, K. Size-controlled one-dimensional monocrystalline BaTiO3 nanostructures. Appl. Phys. Lett. 2009, 94, 253109. [Google Scholar] [CrossRef]
- Adireddy, S.; Lin, C.K.; Cao, B.B.; Zhou, W.L.; Caruntu, G. Solution-Based Growth of Monodisperse Cube-Like BaTiO3 Colloidal Nanocrystals. Chem. Mater. 2010, 22, 1946–1948. [Google Scholar] [CrossRef]
- Mao, Y.B.; Banerjee, S.; Wong, S.S. Hydrothermal synthesis of perovskite nanotubes. Chem. Commun. 2003, 3, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Nakamura, H. Preparation of Hollow BaTiO3 and Anatase Spheres by the Layer-by-Layer Colloidal Templating Method. J. Am. Ceram. Soc. 2006, 89, 1455–1457. [Google Scholar] [CrossRef]
- Ahmad, G.; Dickerson, M.B.; Cai, Y.; Jones, S.E.; Ernst, E.M.; Vernon, J.P.; Haluska, M.S.; Fang, Y.; Wang, J.; Subrarnanyarn, G.; et al. Rapid Bioenabled Formation of Ferroelectric BaTiO3 at Room Temperature from an Aqueous Salt Solution at Near Neutral pH. J. Am. Chem. Soc. 2008, 130, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Nuraje, N.; Su, K.; Haboosheh, A.; Samson, J.; Manning, E.P.; Yang, N.L.; Matsui, H. Room Temperature Synthesis of Ferroelectric Barium Titanate Nanoparticles Using Peptide Nanorings as Templates. Adv. Mater. 2006, 18, 807–811. [Google Scholar] [CrossRef]
- Huang, R.H.; Sobol, N.B.; Younes, A.; Mamun, T.; Lewis, J.S.; Ulijn, R.V.; O’Brien, S. Comparison of Methods for Surface Modification of Barium Titanate Nanoparticles for Aqueous Dispersibility: Toward Biomedical Utilization of Perovskite Oxides. ACS Appl. Mater. Interfaces 2020, 12, 51135–51147. [Google Scholar] [CrossRef]
- Urban, J.J.; Yun, W.S.; Gu, Q.; Park, H. Synthesis of Single-Crystalline Perovskite Nanorods Composed of Barium Titanate and Strontium Titanate. J. Am. Chem. Soc. 2002, 124, 1186–1187. [Google Scholar] [CrossRef]
- Caruntu, D.; Rostamzadeh, T.; Costanzo, T.; Parizia, S.S.; Caruntu, G. Solvothermal synthesis and controlled self-assembly of monodisperse titanium-based perovskite colloidal nanocrystals. Nanoscale 2015, 7, 12955–12969. [Google Scholar] [CrossRef]
- Huang, K.C.; Huang, T.C.; Hsieh, W.F. Morphology-Controlled Synthesis of Barium Titanate Nanostructures. Inorg. Chem. 2009, 48, 9180–9184. [Google Scholar] [CrossRef]
- Demirors, A.F.; Imhof, A. BaTiO3, SrTiO3, CaTiO3, and BaxSr1−xTiO3 Particles: A General Approach for Monodisperse Colloidal Perovskites. Chem. Mater. 2009, 21, 3002–3007. [Google Scholar] [CrossRef]
- Brutchey, R.L.; Morse, D.E. Template-Free, Low-Temperature Synthesis of Crystalline Barium Titanate Nanoparticles under Bio-Inspired Conditions. Angew. Chem. Int. Ed. 2006, 45, 6564–6566. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Hoshina, T.; Ishimura, D.; Kobayashi, S.; Nakamura, W.; Tsurumi, T.; Wada, S. Size effect on the crystal structure of barium titanate nanoparticles. J. Appl. Phys. 2005, 98, 014313. [Google Scholar] [CrossRef]
- Smith, M.B.; Page, K.; Siegrist, T.; Redmond, P.L.; Walter, E.C.; Seshadri, R.; Brus, L.E.; Steigerwald, M.L. Crystal Structure and the Paraelectric-to-Ferroelectric Phase Transition of Nanoscale BaTiO3. J. Am. Chem. Soc. 2008, 130, 6955–6963. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Billinge, S.J.L.; Puma, E.; Bang, S.H.; Bean, N.J.H.; de Sugny, J.-C.; Gambee, R.G.; Haskell, R.C.; Hightower, A.; Monson, T.C. Barium titanate nanoparticles: Short-range lattice distortions with long-range cubic order. Phys. Rev. B 2018, 98, 085421. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T.; Ado, K.; Asai, T.; Kageyama, H.; Saito, Y.; Masquelier, C.; Nakamura, O. Thickness of Cubic Surface Phase on Barium Titanate single crystalline grains. J. Am. Ceram. Soc. 1994, 77, 1665–1668. [Google Scholar] [CrossRef]
- Hoshina, T.; Wada, S.; Kuroiwa, Y.; Tsurumi, T. Composite structure and size effect of barium titanate nanoparticles. Appl. Phys. Lett. 2008, 93, 192914. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Uemori, T. Surface relaxation in ferroelectric perovskites. Phys. Rev. B 1999, 60, 11841–11845. [Google Scholar] [CrossRef]
- Heifets, E.; Dorfman, S.; Fuks, D.; Kotomin, E. Atomistic simulation of the [001] surface structure in BaTiO3. Thin Solid Films 1997, 296, 76–78. [Google Scholar] [CrossRef]
- Merz, W.J. The electric and optical behavior of BatiO3 single-domain crystals. Phys. Rev. 1949, 76, 1221–1225. [Google Scholar] [CrossRef]
- Kojima, A.; Hiraoka, H.; Morimura, R.; Yoshimura, Y.; Tozaki, K. Detailed Evolution of the Ferroelectric Phase Transition in BaTiO3. Ferroelectrics 2014, 462, 129–139. [Google Scholar] [CrossRef]
- Hippel, A. Ferroelectricity domain structure and phase transition of barium titanate. Rev. Mod. Phys. 1950, 22, 221–237. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Rebuffi, L.; Troian, A.; Ciancio, R. On the reliability of powder diffraction Line Profile Analysis of plastically deformed nanocrystalline systems. Sci. Rep. 2016, 6, 20712–20725. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Shen, Z.-G.; Zhang, W.-W.; Chen, J.-F. Size dependence on the ferroelectric transition of nanosized BaTiO3 particles. Mater. Chem. Phys. 2006, 98, 450–455. [Google Scholar] [CrossRef]
- Hsiang, H.-I.; Yen, F.-S. Effect of Crystallite Size on the Ferroelectric Domain Growth of Ultrafine BaTiO3. J. Am. Ceram. Soc. 1996, 79, 1053–1060. [Google Scholar] [CrossRef]
- Barzilay, M.; Elangovan, H.; Ivry, Y. Surface Nucleation of the Paraelectric Phase in Ferroelectric BaTiO3: Atomic Scale Mapping. ACS Appl. Electron. Mater. 2019, 1, 2431–2436. [Google Scholar] [CrossRef] [Green Version]
- Bursill, L.A.; Lin, P.J. Microdomains observed at the ferroelectric/paraelectric phase transition of barium titanate. Nature 1984, 311, 550–552. [Google Scholar] [CrossRef]
- Yoneda, Y.; Kohmura, Y.; Suzuki, Y. X-ray Diffraction Topography of BaTiO3 at Phase Transition Temperature. Jpn. J. Appl. Phys. 2009, 48, 09KF01. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.J.C. X-Ray Optics, 2nd ed.; Methuen &Co Ltd.: New York, NY, USA; John Wiley & Sons Inc: London, UK, 1962. [Google Scholar]
- Leonardi, A.; Leoni, M.; Siboni, S.; Scardi, P. Common volume functions and diffraction line profiles of polyhedral domains. J. Appl. Crystallogr. 2012, 45, 1162–1172. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- DiDomenico, M., Jr.; Wemple, S.H.; Porto, S.P.S.; Bauman, R.P. Raman Spectrum of Single-Domain BaTiO3. Phys. Rev. 1968, 174, 522–530. [Google Scholar] [CrossRef]
- Dobal, P.S.; Dixit, A.; Katiyar, R.S.; Yu, Z.; Guo, R.; Bhalla, A.S. Micro-Raman scattering and dielectric investigations of phase transition behavior in the BaTiO3–BaZrO3 system. J. Appl. Phys. B 2001, 89, 8085–8091. [Google Scholar] [CrossRef]
- Busca, G.; Ramis, G.; Gallardo Amores, J.M.; Sanchez Escribano, V.; Piaggio, P. FT Raman and FTIR studies of titanias and metatitanate powders. J. Chem. Soc. Faraday Trans. 1994, 90, 3181–3190. [Google Scholar] [CrossRef]
- Wada, S.; Suzuki, T.; Osada, M.; Kakihana, M.; Noma, T. Change of Macroscopic and Microscopic Symmetry of Barium Titanate Single Crystal around Curie Temperature. Jpn. J. Appl. Phys. 1998, 37, 5385–5393. [Google Scholar] [CrossRef]
- Perry, C.H.; Hall, D.B. Temperature Dependence of the Raman Spectrum of BaTiO3. Phys. Rev. Lett. 1965, 15, 700–702. [Google Scholar] [CrossRef]
- Busca, G.; Buscaglia, V.; Leoni, M.; Nanni, P. Solid-State and Surface Spectroscopic Characterization of BaTiO3 Fine Powders. Chem. Mater. 1994, 6, 955–961. [Google Scholar] [CrossRef]
- Shiratori, Y.; Pithan, C.; Dornseiffer, J.; Waser, R. Raman scattering studies on nanocrystalline BaTiO3. Part I—Isolated particles and aggregates. J. Raman Spectrosc. 2007, 38, 1288–1299. [Google Scholar] [CrossRef]
- Rabuffetti, F.A.; Brutchey, R.L. Structural Evolution of BaTiO3 Nanocrystals Synthesized at Room Temperature. J. Am. Chem. Soc. 2012, 134, 9475–9487. [Google Scholar] [CrossRef] [PubMed]
- Robins, L.H.; Kaiser, D.L.; Rotter, L.D.; Schenck, P.K.; Stauf, G.T.; Rytz, D. Investigation of the structure of barium titanate thin films by Raman spectroscopy. J. Appl. Phys. 1994, 76, 7487–7498. [Google Scholar] [CrossRef]
- Lee, H.-W.; Moon, S.; Choi, C.-H.; Kim, D.K. Synthesis and Size Control of Tetragonal Barium Titanate Nanopowders by Facile Solvothermal Method. J. Am. Ceram. Soc. 2012, 95, 2429–2434. [Google Scholar] [CrossRef]














| Sample | Crystal System | Crystallite (Domain) Size (nm) Min-Max 1 | Microstrain (arb. units) | Lattice Constant (Å) |
|---|---|---|---|---|
| BTO-3h | Cubic | 18.0 17.8–23.3 | 0.24 | 4.0264 |
| BTO-18h | Cubic | 23.9 22.9–31.7 | 0.29 | 4.0246 |
| BTO-m | Tetrag. | 118 | 0.16 | a = 3.9964 c = 4.0320 |
| Sample | Crystallite Size (nm) | Linear Thermal Expansion Coefficient (10−6 K−1) | Thermal Expansion (%) | Increase in Crystallite Size (%) |
|---|---|---|---|---|
| BTO-3h | 18.0 | 4.8 ± 5% | 0.05 | 0.8 |
| BTO-18h | 23.9 | 3.4 ± 9% | 0.04 | 1.7 |
| BTO-m | 118 | 1.0 ± 80% tetragonal phase 16 ± 10% cubic phase | 0.01 | 22 (up to 110 °C) |
| Reflection (Cubic Lattice) | Cubes (Bounded by {100} Faces) | Octahedrons (Bounded by {111} Faces) |
|---|---|---|
| 100 | 1.0000 | 1.1006 |
| 110 | 1.0607 | 1.0376 |
| 111 | 1.1547 | 1.1438 |
| 210 | 1.0733 | 1.1075 |
| 211 | 1.1527 | 1.1061 |
| 221 | 1.1429 | 1.1185 |
| 310 | 1.0672 | 1.1138 |
| 311 | 1.1359 | 1.1211 |
| Sample Direction | Size (nm) | Microstrain (a.u.) |
|---|---|---|
| BTO-3h | ||
| <100> | 23.3 | 0.54 |
| <110> | 20.0 | 0.26 |
| <111> | 22.5 | 0.27 |
| BTO-18h | ||
| <100> | 31.7 | 0.58 |
| <110> | 26.4 | 0.38 |
| <111> | 26.8 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasuk, I.; Neațu, F.; Neațu, Ș.; Florea, M.; Istrate, C.M.; Pintilie, I.; Pintilie, L. Structural Details of BaTiO3 Nano-Powders Deduced from the Anisotropic XRD Peak Broadening. Nanomaterials 2021, 11, 1121. https://doi.org/10.3390/nano11051121
Pasuk I, Neațu F, Neațu Ș, Florea M, Istrate CM, Pintilie I, Pintilie L. Structural Details of BaTiO3 Nano-Powders Deduced from the Anisotropic XRD Peak Broadening. Nanomaterials. 2021; 11(5):1121. https://doi.org/10.3390/nano11051121
Chicago/Turabian StylePasuk, Iuliana, Florentina Neațu, Ștefan Neațu, Mihaela Florea, Cosmin M. Istrate, Ioana Pintilie, and Lucian Pintilie. 2021. "Structural Details of BaTiO3 Nano-Powders Deduced from the Anisotropic XRD Peak Broadening" Nanomaterials 11, no. 5: 1121. https://doi.org/10.3390/nano11051121
APA StylePasuk, I., Neațu, F., Neațu, Ș., Florea, M., Istrate, C. M., Pintilie, I., & Pintilie, L. (2021). Structural Details of BaTiO3 Nano-Powders Deduced from the Anisotropic XRD Peak Broadening. Nanomaterials, 11(5), 1121. https://doi.org/10.3390/nano11051121