Preparation of Fe3O4-Ag Nanocomposites with Silver Petals for SERS Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
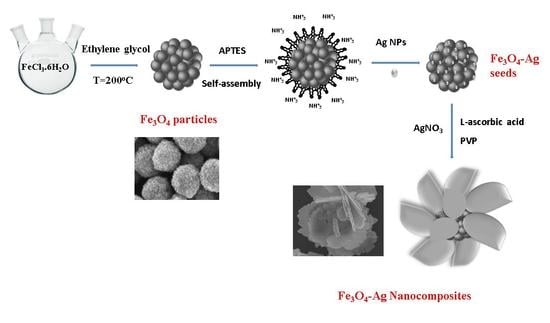
2.2. Synthesis of Fe3O4-Ag Seeds
2.3. Synthesis of Fe3O4-Ag Nanocomposites
2.4. Preparation of SERS Substrates
2.5. Characterizations
3. Results
3.1. Phase and Microstructure Analysis of the Engineered Fe3O4-Ag Nanocomposites
3.2. Application in SERS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Bodelon, G.; Pastoriza-Santos, I. Recent progress in surface-enhanced Raman scattering for the detection of chemical contaminants in water. Front. Chem. 2020, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Gaidi, M.; Daoudi, K.; Tlili, A.; Columbus, S.; Leblanc-Lavoie, J.; Ramachandran, K.; Suleiman, B.; Alhazaa, A.; El Khakani, M. Fast, highly sensitive and label free detection of small genetic sequence difference of DNA using novel Surface-Enhanced Raman Spectroscopy nanostructured sensor. Sens. Bio-Sens. Res. 2021, 32, 100406. [Google Scholar]
- Guselnikova, O.; Postnikova, P.; Marque, S.R.A.; Švorčík, V.; Lyutakov, O. Beyond common analytical limits of radicals detection using the functional SERS substrates. Sens. Actuators B Chem. 2019, 300, 127015. [Google Scholar] [CrossRef]
- Bruzas, I.; Lum, W.; Gorunmez, Z.; Sagle, L. Advances in surface-enhanced Raman spectroscopy (SERS) substrates for lipid and protein characterization: Sensing and beyond. Analyst 2018, 143, 3990–4008. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Langer, J.; Jiménez de Aberasturia, D.; Liz-Marzán, L.M. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahana, A.E.; Byrne, H.J. Surface Enhanced Raman Scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef] [Green Version]
- Onaciu, A.; Braicu, C.; Zimta, A.-A.; Moldovan, A.; Stiufiuc, R.; Buse, M.; Ciocan, C.; Buduru, S.; Berindan-Neagoe, I. Gold nanorods: From anisotropy to opportunity. An evolution update. Nanomedicine 2019, 14. [Google Scholar] [CrossRef]
- Fales, A.M.; Vo-Dinh, T. Silver embedded nanostars for SERS with internal reference (SENSIR). J. Mater. Chem. C 2015, 3, 7319–7324. [Google Scholar] [CrossRef]
- Chen, S.; Carroll, D.L. Synthesis and characterization of truncated triangular silver nanoplates. Nano Lett. 2002, 2, 1003–1007. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Kumar, G.; Soni, R. Silver nanocube-and nanowire-based SERS substrates for ultra-low detection of PATP and thiram molecules. Plasmonics 2020, 15, 1577–1589. [Google Scholar] [CrossRef]
- Aliyah, K.; Lyu, J.; Goldmann, C.; Bizien, T.; Hamon, C.; Alloyeau, D.; Constantin, D. Real-time in situ observations reveal a double role for ascorbic acid in the anisotropic growth of silver on gold. J. Phys. Chem. Lett. 2020, 11, 2830–2837. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Sambri, L.; Tortorella, S.; Šturm, S.; Kostevšek, N.; Comes Franchini, M. Synthesis of ultrasmall single-crystal gold–silver alloy nanotriangles and their application in photothermal therapy. Nanomaterials 2021, 11, 912. [Google Scholar] [CrossRef]
- Qiu, Y.; Deng, D.; Deng, Q.; Wu, P.; Zhang, H.; Cai, C. Synthesis of magnetic Fe3O4-Au hybrids for sensitive SERS detection of cancer cells at low abundance. J. Mater. Chem. B 2015, 3, 4487–4495. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, C.; Wang, J.; Sun, Z.; Xiao, R.; Wang, S. Fe3O4@Ag magnetic nanoparticles for microRNA capture and duplex-specific nuclease signal amplification based SERS detection in cancer cells. Biosens. Bioelectron. 2016, 79, 574–580. [Google Scholar] [CrossRef]
- Wang, C.; Xu, S.; Zhang, K.; Li, M.; Li, Q.; Xiao, R.; Wang, S. Streptomycin-modified Fe3O4-Au@Ag core-satellite magnetic nanoparticles as an effective antibacterial agent. J. Mater. Sci. 2016, 52, 1357–1368. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zou, B.; Gao, T.; Zhang, X.; Du, Z.; Zhou, S. Magnetic-based silver composite microspheres with nanosheet-assembled shell for effective SERS substrate. J. Mater. Chem. C 2013, 1, 2441–2447. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, J.; Ding, Q.; Lin, D.; Dong, R.; Yang, L.; Liu, J. Sea-urchin-like Fe3O4@C@Ag particles: An efficient SERS substrate for detection of organic pollutants. Nanoscale 2013, 5, 5887–5895. [Google Scholar] [CrossRef]
- Kavyani, S.; Baharfar, R. Design and characterization of Fe3O4/GO/Au-Ag nanocomposite as an efficient catalyst for the green synthesis of spirooxindole-dihydropyridines. Appl. Organomet. Chem. 2020, 34, e5560. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, C.; Xiao, G.; Wei, G.; Li, L.; Liu, C.; Su, H. Controlled synthesis and photocatalysis of sea urchin-like Fe3O4@TiO2@Ag nanocomposites. Nanoscale 2016, 8, 5313–5326. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mammeri, F.; Ammar, S. Iron oxide and gold based magneto-plasmonic nanostructures for medical applications: A review. Nanomaterials 2018, 8, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohse, S.E.; Burrows, N.D.; Scarabelli, L.; Liz-Marzán, L.M.; Murphy, C.J. Anisotropic noble metal nanocrystal growth: The role of halides. Chem. Mater. 2014, 26, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Mulvihill, M.J.; Ling, X.Y.; Henzie, J.; Yang, P. Anisotropic etching of silver nanoparticles for plasmonic structures capable of single-particle SERS. J. Am. Chem. Soc. 2010, 132, 268–274. [Google Scholar] [CrossRef]
- Wang, W.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.-J. Monodispersed Core–Shell Fe3O4@Au nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Adams, S.A.; López-Luke, T.; Torres-Castro, A.; Zhang, J.Z. Magnetic Fe3O4-Au core-shell nanostructures for surface enhanced Raman scattering. Ann. Phys. 2012, 524, 670–679. [Google Scholar] [CrossRef]
- Zheng, H.; Zou, B.; Chen, L.; Wang, Y.; Zhang, X.; Zhou, S. Gel-assisted synthesis of oleate-modified Fe3O4@Ag composite microspheres as magnetic SERS probe for thiram detection. CrystEngComm 2015, 17, 6393–6398. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Lu, X.; Duan, W.; Moriga, T. Synthesis and evaluation of the SERS effect of Fe3O4-Ag Janus composite materials for separable, highly sensitive substrates. RSC Adv. 2019, 9, 2877–2884. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, C.; Rong, Z.; Xiao, R.; Zhou, Z.; Wang, S. Silver coated magnetic microflowers as efficient and recyclable catalysts for catalytic reduction. New J. Chem. 2017, 41, 14199–14208. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lau-Truong, S.; Mammeri, F.; Ammar, S. Star-shaped Fe3-xO4-Au core-shell nanoparticles: From synthesis to SERS application. Nanomaterials 2020, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Liang, G.; Jin, W.; Jian, Z.; Wu, Z.; Chen, Q.; Cai, Y.; Zhang, W. Preparation of triangular silver nanoplates by silver seeds capped with citrate-CTA+. RSC Adv. 2018, 8, 28934–28943. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Sun, L.; Shan, Y.; Zhi, Y.; Chen, J.; Dou, B.; Su, W. Green preparation of silver nanofilms as SERS-active substrates for Rhodamine 6G detection. Vacuum 2021, 187, 110096. [Google Scholar] [CrossRef]
- Flores-Romero, E.; Rodriguez-Sevilla, E.; Cheang-Wong, J.C. Silver films over silica microsphere (AgFOSM) as SERS substrates. Photonics Nanostruct. 2018, 28, 81–87. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Mammeri, F.; Ammar, S.; Nguyen, T.B.N.; Nguyen, T.N.; Nghiem, T.H.L.; Thuy, N.T.; Ho, T.A. Preparation of Fe3O4-Ag Nanocomposites with Silver Petals for SERS Application. Nanomaterials 2021, 11, 1288. https://doi.org/10.3390/nano11051288
Nguyen TT, Mammeri F, Ammar S, Nguyen TBN, Nguyen TN, Nghiem THL, Thuy NT, Ho TA. Preparation of Fe3O4-Ag Nanocomposites with Silver Petals for SERS Application. Nanomaterials. 2021; 11(5):1288. https://doi.org/10.3390/nano11051288
Chicago/Turabian StyleNguyen, Thi Thuy, Fayna Mammeri, Souad Ammar, Thi Bich Ngoc Nguyen, Trong Nghia Nguyen, Thi Ha Lien Nghiem, Nguyen Thi Thuy, and Thi Anh Ho. 2021. "Preparation of Fe3O4-Ag Nanocomposites with Silver Petals for SERS Application" Nanomaterials 11, no. 5: 1288. https://doi.org/10.3390/nano11051288
APA StyleNguyen, T. T., Mammeri, F., Ammar, S., Nguyen, T. B. N., Nguyen, T. N., Nghiem, T. H. L., Thuy, N. T., & Ho, T. A. (2021). Preparation of Fe3O4-Ag Nanocomposites with Silver Petals for SERS Application. Nanomaterials, 11(5), 1288. https://doi.org/10.3390/nano11051288