Use of Protamine in Nanopharmaceuticals—A Review
Abstract
:1. Introduction
2. Protamine-Structural Features and Function
2.1. Structural Features
2.2. Molecular Function of Protamine
2.3. Protamine Derivatives
3. Protamine in Various Pharmaceutical Fields
3.1. Protamine in Insulin Preparations
3.2. Protamine-Haemostatic Properties
4. Protamine as Peptide-Based Drug Delivery System
4.1. Cell Penetrating Peptides (CPPs)
4.2. Game Changing Nanotechnology and Protamine’s Approach in this Novel Field
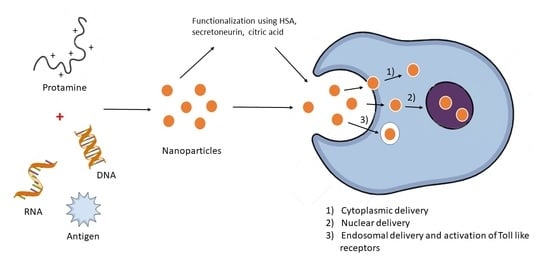
4.2.1. Manufacturing Protamine-Based Nanoparticles
4.2.2. Functionalizing Proticles
4.2.3. Immunogenic Properties of Proticles
5. Protamine and New Vaccine Technologies
5.1. Key Components of the Immune System
Immune Response after Vaccination
5.2. Novel Vaccine Technologies
A Brief History of Vaccinology
5.3. Adjuvants—Components to Boost the Immune Response
5.3.1. Mechanism of Action
Formation of Depot at the Site of Injection
Recruitment of Immune Cells
Enhanced Antigen Uptake and Antigen Presentation
Cytokine and Chemokine Induction
5.4. Nanoparticles as Vaccine Delivery Vehicles
5.4.1. Inorganic Nanoparticles
5.4.2. Liposomes
5.4.3. Virus-Like Particles (VLPs)
5.4.4. Biodegradable Polymeric Nanoparticles (NPs)
5.4.5. Cell-Penetrating Peptides
5.5. Protamine in Vaccine Development
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miescher, F. Das Protamin, Eine Neue Organische Base Aus Den Samenfäden Des Rheinlachses. Ber. Dtsch. Chem. Ges. 1874, 7, 376–379. [Google Scholar] [CrossRef] [Green Version]
- Veigl, S.J.; Harman, O.; Lamm, E. Friedrich Miescher’s Discovery in the Historiography of Genetics: From Contamination to Confusion, from Nuclein to DNA. J. Hist. Biol. 2020, 53, 451–484. [Google Scholar] [CrossRef] [PubMed]
- Morkowin, N. Ein Beitrag zur Kenntniss der Protamine. Hoppe-Seyler’s Z. Physiol. Chem. 1899, 28, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Balhorn, R. The Protamine Family of Sperm Nuclear Proteins. Genome Biol. 2007, 8. [Google Scholar] [CrossRef]
- Kossel, A. Weitere Mittheilungen über die Protamine. Hoppe-Seyler’s Z. Physiol. Chem. 1899, 26, 588–592. [Google Scholar] [CrossRef]
- Ando, T.; Yamasaki, M.; Suzuki, K. Protamines. Isolation, Characterisation, Structure and Function. Mol. Biol. Biochem. Biophys. 1973, 16, 173. [Google Scholar] [CrossRef]
- Vilfan, I.D.; Conwell, C.C.; Hud, N.V. Formation of Native-like Mammalian Sperm Cell Chromatin with Folded Bull Protamine. J. Biol. Chem. 2004, 279, 20088–20095. [Google Scholar] [CrossRef] [Green Version]
- Sorgi, F.L.; Bhattacharya, S.; Huang, L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997, 4, 961–968. [Google Scholar] [CrossRef]
- He, H.; Ye, J.; Liu, E.; Liang, Q.; Liu, Q.; Yang, V.C. Low molecular weight protamine (LMWP): A nontoxic protamine substitute and an effective cell-penetrating peptide. J. Control. Release 2014, 193, 63–73. [Google Scholar] [CrossRef]
- Hagedorn, H.C. Protamine Insulinate. Proc. R. Soc. Med. 1937, 30, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.R. Insulin Preparations with Prolonged Effect. Diabetes Technol. Ther. 2011, 13 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Lindblad, B. Protamine sulphate: A review of its effects: Hypersensitivity and toxicity. Eur. J. Vasc. Surg. 1989, 3, 195–201. [Google Scholar] [CrossRef]
- He, H.; Ye, J.; Liu, E.; Liang, Q.; Liu, Q.; Yang, V.C. Low Molecular Weight Protamine: A Potential Nontoxic Heparin Antagonist. Thromb. Res. 1999, 94, 53–61. [Google Scholar] [CrossRef]
- Sokolowska, E.; Kalaska, B.; Miklosz, J.; Mogielnicki, A. The toxicology of heparin reversal with protamine: Past, present and future. Expert Opin. Drug Metab. Toxicol. 2016, 12, 897–909. [Google Scholar] [CrossRef]
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and Side-Effects of Protamine in Cardiac Surgery: A Narrative Review. Br. J. Anaesth. 2018, 120, 914–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.J.; Chang, L.; Liang, J.F.; Moon, C.; Chung, C.; Yang, V.C. Nontoxic membrane translocation peptide from protamine, low molecular weight protamine (LMWP), for enhanced intracellular protein delivery: In vitro and in vivo study. FASEB J. 2005, 19, 1555–1557. [Google Scholar] [CrossRef]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Chugh, A.; Eudes, F.; Shim, Y.S. Cell-Penetrating Peptides: Nanocarrier for Macromolecule Delivery in Living Cells. IUBMB Life 2010, 62, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lee, J.Y.; Suh, J.S.; Kwon, Y.M.; Lee, S.J.; Chung, J.K.; Lee, D.S.; Yang, V.C.; Chung, C.P.; Park, Y.J. The Systemic Delivery of SiRNAs by a Cell Penetrating Peptide, Low Molecular Weight Protamine. Biomaterials 2010, 31, 1429–1443. [Google Scholar] [CrossRef]
- David, A.E.; Gong, J.; Chertok, B.; Domszy, R.C.; Moon, C.; Park, Y.S.; Wang, N.S.; Yang, A.J.; Yang, V.C. Immobilized Thermolysin for Highly Efficient Production of Low-Molecular-Weight Protamine—An Attractive Cell-Penetrating Peptide for Macromolecular Drug Delivery Applications. J. Biomed. Mater. Res. Part A 2012, 100A, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Bashyal, S.; Noh, G.; Keum, T.; Choi, Y.W.; Lee, S. Cell Penetrating Peptides as an Innovative Approach for Drug Delivery; Then, Present and the Future. J. Pharm. Investig. 2016, 46, 205–220. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochmann, D.; Jauk, E.; Zimmer, A. Drug delivery of oligonucleotides by peptides. Eur. J. Pharm. Biopharm. 2004, 58, 237–251. [Google Scholar] [CrossRef]
- Delgado, D.; Gascón, A.R.; Del Pozo-Rodríguez, A.; Echevarría, E.; Ruiz de Garibay, A.P.; Rodríguez, J.M.; Solinís, M.Á. Dextran-Protamine-Solid Lipid Nanoparticles as a Non-Viral Vector for Gene Therapy: In Vitro Characterization and in Vivo Transfection after Intravenous Administration to Mice. Int. J. Pharm. 2012, 425, 35–43. [Google Scholar] [CrossRef]
- He, S.N.; Li, Y.L.; Yan, J.J.; Zhang, W.; Du, Y.Z.; Yu, H.Y.; Hu, F.Q.; Yuan, H. Ternary Nanoparticles Composed of Cationic Solid Lipid Nanoparticles, Protamine, and DNA for Gene Delivery. Int. J. Nanomed. 2013, 8, 2859. [Google Scholar] [CrossRef] [Green Version]
- Scheicher, B.; Schachner-Nedherer, A.-L.; Zimmer, A. Protamine–oligonucleotide-nanoparticles: Recent advances in drug delivery and drug targeting. Eur. J. Pharm. Sci. 2015, 75, 54–59. [Google Scholar] [CrossRef]
- Scheicher, B.; Lorenzer, C.; Gegenbauer, K.; Partlic, J.; Andreae, F.; Kirsch, A.H.; Rosenkranz, A.R.; Werzer, O.; Zimmer, A. Manufacturing of a Secretoneurin Drug Delivery System with Self-Assembled Protamine Nanoparticles by Titration. PLoS ONE 2016, 11, e0164149. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application: A Review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Fresacher, K.; Helbok, A.; Reiser, M.; Blass, S.; Rangger, C.; Mair, C.; von Guggenberg, E.; Decristoforo, C.; Andreae, F.; Zimmer, A. Comparison of PEGylated and Non-PEGylated Proticles: An in Vitro and in Vivo Study. Eur. J. Pharm. Sci. 2019, 139, 105063. [Google Scholar] [CrossRef] [PubMed]
- Vahedifard, F.; Chakravarthy, K. Nanomedicine for COVID-19: The role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater. 2021, 4, 75–99. [Google Scholar] [CrossRef]
- Kang, S.-M.; Compans, R.W. Host responses from innate to adaptive immunity after vaccination: Molecular and cellular events. Mol. Cells 2009, 27, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Chapter 1. Introduction to the Immune Response; Harvard University Press: Cambridge, MA, USA, 2013; pp. 1–11. [Google Scholar] [CrossRef]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A. Nucleic Acid Nanoparticles at a Crossroads of Vaccines and Immunotherapies. Molecules 2019, 24, 4620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrie, Y.; Crofts, F.; Devitt, A.; Griffiths, H.R.; Kastner, E.; Nadella, V. Designing liposomal adjuvants for the next generation of vaccines. Adv. Drug Deliv. Rev. 2016, 99, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Frietze, K.M.; Peabody, D.S.; Chackerian, B. Engineering Virus-like Particles as Vaccine Platforms. Curr. Opin. Virol. 2016, 18, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Gregory, A.E.; Etitball, R.; Ewilliamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skwarczynski, M.; Toth, I. Cell-penetrating peptides in vaccine delivery: Facts, challenges and perspectives. Ther. Deliv. 2019, 10, 465–467. [Google Scholar] [CrossRef] [Green Version]
- Scheel, B.; Teufel, R.; Probst, J.; Carralot, J.-P.; Geginat, J.; Radsak, M.; Jarrossay, D.; Wagner, H.; Rammensee, H.-G.; Hoerr, I.; et al. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur. J. Immunol. 2005, 35, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Fotin-Mleczek, M.; Duchardt, K.M.; Lorenz, C.; Pfeiffer, R.; Ojkić-Zrna, S.; Probst, J.; Kallen, K.-J. Messenger RNA-Based Vaccines with Dual Activity Induce Balanced TLR-7 Dependent Adaptive Immune Responses and Provide Antitumor Activity. J. Immunother. 2011, 34, 1–15. [Google Scholar] [CrossRef]
- González-Aramundiz, J.V.; Presas, E.; Dalmau-Mena, I.; Martínez-Pulgarín, S.; Alonso, C.; Escribano, J.M.; Alonso, M.J.; Csaba, N.S. Rational design of protamine nanocapsules as antigen delivery carriers. J. Control. Release 2017, 245, 62–69. [Google Scholar] [CrossRef]
- Kerkmann, M.; Lochmann, D.; Weyermann, J.; Marschner, A.; Poeck, H.; Wagner, M.; Battiany, J.; Zimmer, A.; Endres, S.; Hartmann, G. Immunostimulatory Properties of CpG-Oligonucleotides Are Enhanced by the Use of Protamine Nanoparticles. Oligonucleotides 2006, 16, 313–322. [Google Scholar] [CrossRef]
- Gómez, J.M.M.; Fischer, S.; Csaba, N.; Kündig, T.M.; Merkle, H.P.; Gander, B.; Johansen, P. A Protective Allergy Vaccine Based on CpG- and Protamine-Containing PLGA Microparticles. Pharm. Res. 2007, 24, 1927–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pali-Schöll, I.; Szöllösi, H.; Starkl, P.; Scheicher, B.; Stremnitzer, C.; Hofmeister, A.; Roth-Walter, F.; Lukschal, A.; Diesner, S.C.; Zimmer, A.; et al. Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur. J. Pharm. Biopharm. 2013, 85, 656–664. [Google Scholar] [CrossRef]
- González-Aramundiz, J.; Olmedo, M.P.; González-Fernández, Á.; Fernández, M.J.A.; Csaba, N.S. Protamine-based nanoparticles as new antigen delivery systems. Eur. J. Pharm. Biopharm. 2015, 97, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Dai, S.; Jiao, Y.; Jiang, L.; Zhao, Y.; Wang, B.; Zong, L. Mannosylated protamine as a novel DNA vaccine carrier for effective induction of anti-tumor immune responses. Int. J. Pharm. 2016, 506, 394–406. [Google Scholar] [CrossRef]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef] [PubMed]
- González-Aramundiz, J.; Peleteiro, M.; González-Fernández, A.; Alonso, M.J.; Csaba, N.S.; Olmedo, M.P.; Fernandez, M.J.A. Protamine Nanocapsules for the Development of Thermostable Adjuvanted Nanovaccines. Mol. Pharm. 2018, 15, 5653–5664. [Google Scholar] [CrossRef]
- Vogel, V.; Lochmann, D.; Weyermann, J.; Mayer, G.; Tziatzios, C.; Broek, J.A.V.D.; Haase, W.; Wouters, D.; Schubert, U.S.; Kreuter, J.; et al. Oligonucleotide–protamine–albumin nanoparticles: Preparation, physical properties, and intracellular distribution. J. Control. Release 2005, 103, 99–111. [Google Scholar] [CrossRef]
- Park, Y.J.; Liang, J.F.; Ko, K.S.; Kim, S.W.; Yang, V.C. Low molecular weight protamine as an efficient and nontoxic gene carrier: In vitro study. J. Gene Med. 2003, 5, 700–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junghans, M.; Loitsch, S.M.; Steiniger, S.C.; Kreuter, J.; Zimmer, A. Cationic lipid–protamine–DNA (LPD) complexes for delivery of antisense c-myc oligonucleotides. Eur. J. Pharm. Biopharm. 2005, 60, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, M.; Presas, E.; González-Aramundiz, J.; Sánchez-Correa, B.; Simón-Vázquez, R.; Csaba, N.; Alonso, M.J.; González-Fernández, Á. Polymeric Nanocapsules for Vaccine Delivery: Influence of the Polymeric Shell on the Interaction with the Immune System. Front. Immunol. 2018, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- González-Paredes, A.; Sitia, L.; Ruyra, A.; Morris, C.J.; Wheeler, G.N.; McArthur, M.; Gasco, P. Solid lipid nanoparticles for the delivery of anti-microbial oligonucleotides. Eur. J. Pharm. Biopharm. 2019, 134, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Sköld, A.E.; van Beek, J.; Sittig, S.P.; Bakdash, G.; Tel, J.; Schreibelt, G.; De Vries, I.J.M. Protamine-stabilized RNA as an ex vivo stimulant of primary human dendritic cell subsets. Cancer Immunol. Immunother. 2015, 64, 1461–1473. [Google Scholar] [CrossRef] [Green Version]
- Bench, G.S.; Friz, A.M.; Corzett, M.H.; Morse, D.H.; Balhorn, R. DNA and Total Protamine Masses in Individual Sperm from Fertile Mammalian Subjects. Cytometry 1996, 23, 263–271. [Google Scholar] [CrossRef]
- Bench, G.; Corzett, M.H.; Kramer, C.E.; Grant, P.G.; Balhorn, R. Zinc Is Sufficiently Abundant within Mammalian Sperm Nuclei to Bind Stoichiometrically with Protamine 2. Mol. Reprod. Dev. 2000, 56, 512–519. [Google Scholar] [CrossRef]
- Hutchison, J.M.; Rau, D.C.; DeRouchey, J.E. Role of Disulfide Bonds on DNA Packaging Forces in Bull Sperm Chromatin. Biophys. J. 2017, 113, 1925–1933. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.R.; Thompson, M.B.; Attaway, M.B.; Herbert, J.F.; Murphy, C.R. Of the Chorioallantoic Placentome and the Omphalopleure of the Placentotrophic Lizard, Pseudemoia Entrecasteauxii. J. Exp. Zool. Part A-Comp. Exp. Biol. 2006, 305A, 883–889. [Google Scholar] [CrossRef]
- Ausió, J. Histone H1 and Evolution of Sperm Nuclear Basic Proteins. J. Biol. Chem. 1999, 274, 31115–31118. [Google Scholar] [CrossRef] [Green Version]
- Raukas, E.; Mikelsaar, R.H. Are There Molecules of Nucleoprotamine? BioEssays 1999, 21, 440–448. [Google Scholar] [CrossRef]
- Pogany, G.C.; Corzett, M.; Weston, S.; Balhorn, R. DNA and protein content of mouse sperm: Implications regarding sperm chromatin structure. Exp. Cell Res. 1981, 136, 127–136. [Google Scholar] [CrossRef]
- Teif, V.B.; Bohinc, K. Condensed DNA: Condensing the concepts. Prog. Biophys. Mol. Biol. 2011, 105, 208–222. [Google Scholar] [CrossRef]
- González-Rojo, S.; Fernández-Díez, C.; Guerra, S.M.; Robles, V.; Herráez, M.P. Differential Gene Susceptibility to Sperm DNA Damage: Analysis of Developmental Key Genes in Trout. PLoS ONE 2014, 9, e114161. [Google Scholar] [CrossRef]
- Nili, H.A.; Mozdarani, H.; Aleyasin, A. Correlation of sperm DNA damage with protamine deficiency in Iranian subfertile men. Reprod. Biomed. Online 2009, 18, 479–485. [Google Scholar] [CrossRef]
- Prieto, M.C.; Maki, A.H.; Balhorn, R. Analysis of DNA-Protamine Interactions by Optical Detection of Magnetic Resonance. Biochemistry 1997, 36, 11944–11951. [Google Scholar] [CrossRef] [PubMed]
- Hud, N.V.; Downing, K.H. Cryoelectron microscopy of phage DNA condensates in vitreous ice: The fine structure of DNA toroids. Proc. Natl. Acad. Sci. USA 2001, 98, 14925–14930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hud, N.; Allen, M.; Downing, K.; Lee, J.; Balhorn, R. Identification of the Elemental Packing Unit of DNA in Mammalian Sperm Cells by Atomic Force Microscopy. Biochem. Biophys. Res. Commun. 1993, 193, 1347–1354. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, P.; Hsiao, P.-Y. Stick-Release Pattern in Stretching Single Condensed Polyelectrolyte Toroids. Macromolecules 2009, 42, 3211–3214. [Google Scholar] [CrossRef] [Green Version]
- Ukogu, O.A.; Smith, A.D.; Devenica, L.M.; Bediako, H.; McMillan, R.B.; Ma, Y.; Balaji, A.; Schwab, R.D.; Anwar, S.; Dasgupta, M.; et al. Protamine loops DNA in multiple steps. Nucleic Acids Res. 2020, 48, 6108–6119. [Google Scholar] [CrossRef] [PubMed]
- Belokopytova, I.A.; Kostyleva, E.I.; Tomilin, A.N.; Vorob’ev, V.I. Human Male Infertility May Be Due to a Decrease of the Protamine P2 Content in Sperm Chromatin. Mol. Reprod. Dev. 1993, 34, 53–57. [Google Scholar] [CrossRef]
- Rahme, K.; Guo, J.; Holmes, J.D.; O’Driscoll, C.M. Evaluation of the physicochemical properties and the biocompatibility of polyethylene glycol-conjugated gold nanoparticles: A formulation strategy for siRNA delivery. Colloids Surf. B Biointerfaces 2015, 135, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Lee, H.F.; Yang, Z.; Yang, V.C. Low Molecular Weight Protamine (LMWP) as Nontoxic Heparin/Low Molecular Weight Heparin Antidote (I): Preparation and Characterization. AAPS PharmSci. 2001, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Ye, J.; Wang, Y.; Liu, Q.; Chung, H.S.; Kwon, Y.M.; Shin, M.C.; Lee, K.; Yang, V.C. Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J. Control. Release 2014, 176, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Junghans, M. Antisense delivery using protamine-oligonucleotide particles. Nucleic Acids Res. 2000, 28, E45. [Google Scholar] [CrossRef]
- Lochmann, D.; Weyermann, J.; Georgens, C.; Prassl, R.; Zimmer, A. Albumin–protamine–oligonucleotide nanoparticles as a new antisense delivery system. Part 1: Physicochemical characterization. Eur. J. Pharm. Biopharm. 2005, 59, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Vogel, V.; Weyermann, J.; Lochmann, D.; Broek, J.A.V.D.; Tziatzios, C.; Haase, W.; Wouters, D.; Schubert, U.S.; Zimmer, A.; et al. Oligonucleotide-protamine-albumin nanoparticles: Protamine sulfate causes drastic size reduction. J. Control. Release 2005, 106, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Dinauer, N.; Lochmann, D.; Demirhan, I.; Bouazzaoui, A.; Zimmer, A.; Chandra, A.; Kreuter, J.; von Briesen, H. Intracellular Tracking of Protamine/Antisense Oligonucleotide Nanoparticles and Their Inhibitory Effect on HIV-1 Transactivation. J. Control. Release 2004, 96, 497–507. [Google Scholar] [CrossRef]
- Kallen, K.-J.; Heidenreich, R.; Schnee, M.; Petsch, B.; Schlake, T.; Thess, A.; Baumhof, P.; Scheel, B.; Koch, S.D.; Fotin-Mleczek, M. A novel, disruptive vaccination technology. Hum. Vaccines Immunother. 2013, 9, 2263–2276. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Giesslinger, G.; Menzel, S.; Kroemer, H.K.; Ruth, P.; Mutschler, E. Mutschler Arzneimittelwirkungen Pharmakologie Klinische Pharmakologie Toxikologie, 10th ed.; Wissenschaftliche Verlagsgesellschaft Stuttgart: Stuttgart, Germany, 2012. [Google Scholar]
- Ranasinghe, T.; Mays, T.; Quedado, J.; Adcock, A. Thrombolysis Following Heparin Reversal with Protamine Sulfate in Acute Ischemic Stroke: Case Series and Literature Review. J. Stroke Cerebrovasc. Dis. 2019, 28, 104283. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, J.; Lin, Y.A.; Prielipp, R.C.; Bennett, J.; Hammon, J.W.; James, R.L. Rapid Disappearance of Protamine in Adults Undergoing Cardiac Operation with Cardiopulmonary Bypass. Ann. Thorac. Surg. 2002, 74, 1589–1595. [Google Scholar] [CrossRef]
- Schroeder, M.; Hogwood, J.; Gray, E.; Mulloy, B.; Hackett, A.-M.; Johansen, K.B. Protamine neutralisation of low molecular weight heparins and their oligosaccharide components. Anal. Bioanal. Chem. 2011, 399, 763–771. [Google Scholar] [CrossRef]
- Hecht, P.; Besser, M.; Falter, F. Are We Able to Dose Protamine Accurately Yet? A Review of the Protamine Conundrum. J. Extra-Corpor. Technol. 2020, 52, 63–70. [Google Scholar] [CrossRef]
- Nybo, M.; Madsen, J.S. Serious Anaphylactic Reactions due to Protamine Sulfate: A Systematic Literature Review. Basic Clin. Pharmacol. Toxicol. 2008, 103, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.X.; Garneau-Tsodikova, S. What are the drugs of the future? MedChemComm 2018, 9, 757–758. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef]
- Reissmann, S. Cell penetration: Scope and limitations by the application of cell-penetrating peptides. J. Pept. Sci. 2014, 20, 760–784. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, S.; Morris, M.C.; Divita, G.; Heitz, F. Cell-Penetrating Pep Tides: Tools for Intracellular Delivery of Therapeutics. Cell. Mol. Life Sci. 2005, 62, 1839–1849. [Google Scholar] [CrossRef]
- Munyendo, W.L.; Lv, H.; Benza-Ingoula, H.; Baraza, L.D.; Zhou, J. Cell Penetrating Peptides in the Delivery of Biopharmaceuticals. Biomolecules 2012, 2, 187–202. [Google Scholar] [CrossRef]
- Ruczynski, J.; Wierzbicki, P.M.; Kogut-Wierzbicka, M.; Mucha, P.; Siedlecka-Kroplewska, K.; Rekowski, P. Cell-penetrating peptides as a promising tool for delivery of various molecules into the cells. Folia Histochem. Cytobiol. 2015, 52, 257–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allhoff, F. On the Autonomy and Justification of Nanoethics. NanoEthics 2007, 1, 185–210. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dul, M.; Paluch, K.J.; Kelly, H.; Healy, A.M.; Sasse, A.; Tajber, L. Self-Assembled Carrageenan/Protamine Polyelectrolyte Nanoplexes-Investigation of Critical Parameters Governing Their Formation and Characteristics. Carbohydr. Polym. 2015, 123, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Schachner-Nedherer, A.-L.; Werzer, O.; Kornmueller, K.; Prassl, R.; Zimmer, A. Biological Activity of miRNA-27a Using Peptide-based Drug Delivery Systems. Int. J. Nanomed. 2019, 14, 7795–7808. [Google Scholar] [CrossRef] [Green Version]
- Palazzolo, S.; Hadla, M.; Spena, C.R.; Caligiuri, I.; Rotondo, R.; Adeel, M.; Kumar, V.; Corona, G.; Canzonieri, V.; Toffoli, G.; et al. An Effective Multi-Stage Liposomal DNA Origami Nanosystem for In Vivo Cancer Therapy. Cancers 2019, 11, 1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazzolo, S.; Hadla, M.; Spena, C.R.; Bayda, S.; Kumar, V.; Re, F.L.; Adeel, M.; Caligiuri, I.; Romano, F.; Corona, G.; et al. Proof-of-Concept Multistage Biomimetic Liposomal DNA Origami Nanosystem for the Remote Loading of Doxorubicin. ACS Med. Chem. Lett. 2019, 10, 517–521. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Black, K.C.; Wang, Y.; Luehmann, H.P.; Cai, X.; Xing, W.; Pang, B.; Zhao, Y.; Cutler, C.S.; Wang, L.V.; Liu, Y.; et al. Radioactive 198Au-Doped Nanostructures with Different Shapes for in Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8, 4385–4394. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzer, C.; Dirin, M.; Winkler, A.-M.; Baumann, V.; Winkler, J. Going beyond the liver: Progress and challenges of targeted delivery of siRNA therapeutics. J. Control. Release 2015, 203, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Merkel, T.J.; Chen, K.; Jones, S.W.; Pandya, A.A.; Tian, S.; Napier, M.E.; Zamboni, W.E.; DeSimone, J.M. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J. Control. Release 2012, 162, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Björnmalm, M.; Liang, K.; Xu, C.; Best, J.P.; Zhang, X.; Caruso, F. Super-Soft Hydrogel Particles with Tunable Elasticity in a Microfluidic Blood Capillary Model. Adv. Mater. 2014, 26, 7295–7299. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.B.; Yang, C.; Tan, J.P.; Gao, S.; Williams, D.F.; Hedrick, J.L.; Yang, Y.Y. The Effect of Kinetic Stability on Biodistribution and Anti-Tumor Efficacy of Drug-Loaded Biodegradable Polymeric Micelles. Biomaterials 2013, 34, 3132–3140. [Google Scholar] [CrossRef]
- Fröhlich, E.; Samberger, C.; Kueznik, T.; Absenger, M.; Roblegg, E.; Zimmer, A.; Pieber, T.R. Cytotoxicity of Nanoparticles Independent from Oxidative Stress. J. Toxicol. Sci. 2009, 34, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Patil, G.; Khan, M.I.; Patel, D.K.; Sultana, S.; Prasad, R.; Ahmad, I. Evaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic responses of micro- and nano-particles of dolomite on human lung epithelial cells A 549. Environ. Toxicol. Pharmacol. 2012, 34, 436–445. [Google Scholar] [CrossRef]
- Pietruska, J.R.; Liu, X.; Smith, A.; McNeil, K.; Weston, P.; Zhitkovich, A.; Hurt, R.; Kane, A.B. Bioavailability, Intracellular Mobilization of Nickel, and HIF-1α Activation in Human Lung Epithelial Cells Exposed to Metallic Nickel and Nickel Oxide Nanoparticles. Toxicol. Sci. 2011, 124, 138–148. [Google Scholar] [CrossRef]
- Takagi, A.; Hirose, A.; Nishimura, T.; Fukumori, N.; Ogata, A.; Ohashi, N.; Kitajima, S.; Kanno, J. Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. J. Toxicol. Sci. 2008, 33, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Too, H.; Chow, G. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials 2005, 26, 5818–5826. [Google Scholar] [CrossRef] [PubMed]
- Onuma, K.; Sato, Y.; Ogawara, S.; Shirasawa, N.; Kobayashi, M.; Yoshitake, J.; Yoshimura, T.; Iigo, M.; Fujii, J.; Okada, F. Nano-Scaled Particles of Titanium Dioxide Convert Benign Mouse Fibrosarcoma Cells into Aggressive Tumor Cells. Am. J. Pathol. 2009, 175, 2171–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junghans, M.; Kreuter, J.; Zimmer, A. Phosphodiester and phosphorothioate oligonucleotide condensation and preparation of antisense nanoparticles. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 2001, 1544, 177–188. [Google Scholar] [CrossRef]
- Rajagopal, K.; Schneider, J.P. Self-assembling peptides and proteins for nanotechnological applications. Curr. Opin. Struct. Biol. 2004, 14, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nat. Cell Biol. 2006, 440, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Petschacher, C.; Eitzlmayr, A.; Besenhard, M.; Wagner, J.; Barthelmes, J.; Bernkop-Schnürch, A.; Khinast, J.G.; Zimmer, A. Thinking continuously: A microreactor for the production and scale-up of biodegradable, self-assembled nanoparticles. Polym. Chem. 2013, 4, 2342–2352. [Google Scholar] [CrossRef] [Green Version]
- Elsadek, B.; Kratz, F. Impact of Albumin on Drug Delivery—New Applications on the Horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Cao, Z.; Umek, R.M.; McKnight, S.L. McKnight. Regulated Expression of Three C/EBP Isoforms during Adipose Conversion of 3T3-L1 Cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [Green Version]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-Based Drug Delivery: Harnessing Nature to Cure Disease. Mol. Cell. Ther. 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Harthi, S.; Lachowicz, J.I.; Nowakowski, M.E.; Jaremko, M.; Jaremko, Ł. Towards the Functional High-Resolution Coordination Chemistry of Blood Plasma Human Serum Albumin. J. Inorg. Biochem. 2019, 198, 110716. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the Mysteries of Serum Albumin-More than Just a Serum Protein. Front. Physiol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Tian, H.; Wu, J.; Cheng, J.; Luo, C.; Sai, W.; Song, X.; Gao, X.; Yao, W. C-terminal site-specific PEGylated Exendin-4 analog: A long-acting glucagon like Peptide-1 receptor agonist, on glycemic control and beta cell function in diabetic db/db mice. J. Pharmacol. Sci. 2018, 138, 23–30. [Google Scholar] [CrossRef]
- Shokrzadeh, N.; Winkler, A.-M.; Dirin, M.; Winkler, J. Oligonucleotides conjugated with short chemically defined polyethylene glycol chains are efficient antisense agents. Bioorganic Med. Chem. Lett. 2014, 24, 5758–5761. [Google Scholar] [CrossRef] [Green Version]
- Gaziova, Z.; Baumann, V.; Winkler, A.-M.; Winkler, J. Chemically defined polyethylene glycol siRNA conjugates with enhanced gene silencing effect. Bioorganic Med. Chem. 2014, 22, 2320–2326. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.-M. Cationic Peptide-SiRNA Nanocomplexes with Designed Ankyrin Repeat Proteins for Active Receptor Targeting. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2018. [Google Scholar]
- Dozier, J.K.; Distefano, M.D. Site-Specific Pegylation of Therapeutic Proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Jin, Y.; Chivukula, P.; Zhang, J.; Liu, Y.; Liu, J.; Clamme, J.P.; Mahato, R.I.; Ng, D.; Ying, W.; et al. Effect of PEGylation on Biodistribution and Gene Silencing of SiRNA/Lipid Nanoparticle Complexes. Pharm. Res. 2013, 30, 342–351. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Ogier, J.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Cationic and PEGylated Amphiphilic Cyclodextrins: Co-Formulation Opportunities for Neuronal Sirna Delivery. PLoS ONE 2013, 8, e66413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phonesouk, E.; Lechevallier, S.; Ferrand, A.; Rols, M.-P.; Bezombes, C.; Verelst, M.; Golzio, M. Increasing Uptake of Silica Nanoparticles with Electroporation: From Cellular Characterization to Potential Applications. Materials 2019, 12, 179. [Google Scholar] [CrossRef] [Green Version]
- Iversen, F.; Yang, C.; Dagnæs-Hansen, F.; Schaffert, D.H.; Kjems, J.; Gao, S. Optimized siRNA-PEG Conjugates for Extended Blood Circulation and Reduced Urine Excretion in Mice. Theranostics 2013, 3, 201–209. [Google Scholar] [CrossRef]
- He, X.; Nie, H.; Wang, K.; Tan, W.; Wu, X.; Zhang, P. In Vivo Study of Biodistribution and Urinary Excretion of Surface-Modified Silica Nanoparticles. Anal. Chem. 2008, 80, 9597–9603. [Google Scholar] [CrossRef] [PubMed]
- Cohe, J.S. Designing Antisense Oligonucleotides as Pharmaceutical Agents. Trends Pharm. Sci. 1989, 10, 436–437. [Google Scholar] [CrossRef]
- Lochmann, D. Self Assembly von protaminbasierten Oligonukleotid-Nanopartikeln. Ph.D. Thesis, Johann Wolfgang Goethe-University, Frankfurt, Germany, 2004. [Google Scholar]
- del Pozo-Rodríguez, A.; Solinís, M. Ángeles; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Radtke, M.; Wissing, S. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- del Pozo-Rodríguez, A.; Solinís, M.; Gascón, A.; Pedraz, J. Short- and long-term stability study of lyophilized solid lipid nanoparticles for gene therapy. Eur. J. Pharm. Biopharm. 2009, 71, 181–189. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M.; Gascón, A.; Pedraz, J. Solid lipid nanoparticles: Formulation factors affecting cell transfection capacity. Int. J. Pharm. 2007, 339, 261–268. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, W.; Du, Y.-Z.; Hu, F.-Q. Ternary nanoparticles of anionic lipid nanoparticles/protamine/DNA for gene delivery. Int. J. Pharm. 2010, 392, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Wernig, K.; Griesbacher, M.; Andreae, F.; Hajos, F.; Wagner, J.; Mosgoeller, W.; Zimmer, A. Depot formulation of vasoactive intestinal peptide by protamine-based biodegradable nanoparticles. J. Control. Release 2008, 130, 192–198. [Google Scholar] [CrossRef]
- Ortner, A.; Wernig, K.; Kaisler, R.; Edetsberger, M.; Hajos, F.; Köhler, G.; Mosgoeller, W.; Zimmer, A. VPAC receptor mediated tumor cell targeting by protamine based nanoparticles. J. Drug Target. 2010, 18, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-mediated Transport of Nanoparticle-bound Drugs Across the Blood-Brain Barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, I.; Wernig, K.; Panzenboeck, U.; Bernhart, E.; Reicher, H.; Wronski, R.; Windisch, M.; Hammer, A.; Malle, E.; Zimmer, A.; et al. Apolipoprotein A-I coating of protamine–oligonucleotide nanoparticles increases particle uptake and transcytosis in an in vitro model of the blood–brain barrier. J. Control. Release 2007, 117, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almer, G.; Summers, K.L.; Scheicher, B.; Kellner, J.; Stelzer, I.; Leitinger, G.; Gries, A.; Prassl, R.; Zimmer, A.; Mangge, H. Interleukin 10-Coated Nanoparticle Systems Compared for Molecular Imaging of Atherosclerotic Lesions. Int. J. Nanomed. 2014, 9, 4211–4222. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Tung, C.-H.; Zu, Y. Aptamer-Equipped Protamine Nanomedicine for Precision Lymphoma Therapy. Cancers 2020, 12, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [Green Version]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 1–22. [Google Scholar] [CrossRef]
- Wallis, J.; Shenton, D.P.; Carlisle, R.C. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 2019, 196, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Sattler, S. Cardiovascular Immunology Sattler, AEMB 2017 Chapter 1: The Role of the Immune System beyond the Fight against Infection. Adv. Exp. Med. Biol. 2017, 1003, 3–14. [Google Scholar] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 493–518. [Google Scholar] [CrossRef] [Green Version]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Six, A.; Bellier, B.; Thomas-Vaslin, V.; Klatzmann, D. Systems biology in vaccine design. Microb. Biotechnol. 2011, 5, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Zepp, F. Principles of Vaccination. Adv. Struct. Saf. Stud. 2016, 1403, 57–84. [Google Scholar] [CrossRef]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Choi, J.; Oh, Y.; Cho, Y.; Lee, S. Vaccine Adjuvants: Smart Components to Boost the Immune System. Arch. Pharmacal Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Plotkin, S.L. The development of vaccines: How the past led to the future. Nat. Rev. Genet. 2011, 9, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Fine, P. Science and Society: Vaccines and Public Health. Public Health 2014, 128, 686–692. [Google Scholar] [CrossRef]
- Smith, K.A. Edward Jenner and the Small Pox Vaccine. Front. Immunol. 2011, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Berche, P. Louis Pasteur, from Crystals of Life to Vaccination. Clin. Microbiol. Infect. 2012, 18 (Suppl. 5), 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ebeling, A.H. A Ten Year Old Strain of Fibroblasts. J. Exp. Med. 1922, 35, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, J.A. Dr. Carrel’s immortal cells. Med. Hist. 1980, 24, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Salk, J.E.; Krech, U.; Youngner, J.S.; Bennett, B.L.; Lewis, L.J.; Bazeley, P.L. Formaldehyde Treatment and Safety Testing of Experimental Poliomyelitis Vaccines. Am. J. Public Health Nations Health 1954, 44, 563–570. [Google Scholar] [CrossRef]
- Sabin, A.B.; Hennessen, W.A.; Winsser, J. Studies on variants of poliomyelitis virus. J. Exp. Med. 1954, 99, 551–576. [Google Scholar] [CrossRef] [Green Version]
- Wharton, M.E. Measles Elimination in the United States. J. Infect. Dis. 2004, 189, S1–S3. [Google Scholar] [CrossRef]
- Hilleman, M.R. Past, Present, and Future of Measles, Mumps, and Rubella Virus Vaccines. Pediatrics 1992, 90 Pt 2, 149–153. [Google Scholar]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef]
- Moyle, P.M.; Toth, I. Modern Subunit Vaccines: Development, Components, and Research Opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.M. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol. Adv. 2017, 35, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, P.; Clementi, E.; Radice, S. On vaccine’s adjuvants and autoimmunity: Current evidence and future perspectives. Autoimmun. Rev. 2015, 14, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Malyala, P.; O’Hagan, D.T. Vaccine Adjuvant Formulations: A Pharmaceutical Perspective. Semin. Immunol. 2013, 25, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Akira, S. Innate Immunity and Adjuvants. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2748–2755. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Lousada-Dietrich, S.; Jogdand, P.S.; Jepsen, S.; Pinto, V.V.; Ditlev, S.B.; Christiansen, M.; Larsen, S.O.; Fox, C.B.; Raman, V.S.; Howard, R.F.; et al. A synthetic TLR4 agonist formulated in an emulsion enhances humoral and Type 1 cellular immune responses against GMZ2—A GLURP–MSP3 fusion protein malaria vaccine candidate. Vaccine 2011, 29, 3284–3292. [Google Scholar] [CrossRef]
- Cox, J.C.; Coulter, A.R. Adjuvants—A Classification and Review of Their Modes of Action. Vaccine 1997, 15, 248–256. [Google Scholar] [CrossRef]
- de Apostólico, J.S.; Lunardelli, V.A.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res. 2016. [CrossRef] [Green Version]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Glenny, A.T.; Pope, C.G.; Waddington, H.; Wallace, U. Immunological notes. XVII-XXIV. J. Pathol. Bacteriol. 1926, 29, 31–40. [Google Scholar] [CrossRef]
- Gupta, R.K. In vivo distribution of radioactivity in mice after injection of biodegradable polymer microspheres containing 14C-labeled tetanus toxoid. Vaccine 1996, 14, 1412–1416. [Google Scholar] [CrossRef]
- Hutchison, S.; Benson, R.A.; Gibson, V.B.; Pollock, A.H.; Garside, P.; Brewer, J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2011, 26, 1272–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billiau, A.; Matthys, P. Modes of Action of Freund’s Adjuvants in Experimental Models of Autoimmune Diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Herbert, W.J. Antigenicity of Soluble Protein in the Presence of High Levels of Antibody: A Possible Mode of Action of the Antigen Adjuvants. Nat. Cell Biol. 1966, 210, 747–748. [Google Scholar] [CrossRef]
- Kool, M.; Pétrilli, V.; De Smedt, T.; Rolaz, A.; Hammad, H.; Van Nimwegen, M.; Bergen, I.M.; Castillo, R.; Lambrecht, B.N.; Tschopp, J. Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. J. Immunol. 2008, 181, 3755–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKee, A.S.; Munks, M.W.; MacLeod, M.K.L.; Fleenor, C.J.; Van Rooijen, N.; Kappler, J.W.; Marrack, P. Alum Induces Innate Immune Responses through Macrophage and Mast Cell Sensors, But These Sensors Are Not Required for Alum to Act As an Adjuvant for Specific Immunity. J. Immunol. 2009, 183, 4403–4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabro, S.; Tortoli, M.; Baudner, B.C.; Pacitto, A.; Cortese, M.; O’Hagan, D.T.; De Gregorio, E.; Seubert, A.; Wack, A. Vaccine Adjuvants Alum and MF59 Induce Rapid Recruitment of Neutrophils and Monocytes That Participate in Antigen Transport to Draining Lymph Nodes. Vaccine 2011, 29, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.; Denis-Mize, K.; LaBarbara, A.; Peters, W.; Charo, I.F.; McDonald, D.M.; Ott, G. Immunization with the Adjuvant MF59 Induces Macrophage Trafficking and Apoptosis. Eur. J. Immunol. 2001, 31, 2910–2918. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [Green Version]
- Korsholm, K.S.; Petersen, R.V.; Agger, E.M.; Andersen, P. T-helper 1 and T-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology 2009, 129, 75–86. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Bramwell, V.W.; Christensen, D.; Agger, E.-M.; Andersen, P.; Perrie, Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J. Control. Release 2010, 142, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Mannhalter, J.W.; Neychev, H.O.; Zlabinger, G.; Ahmad, R.; Eibl, M.M. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: Effect on antigen uptake and antigen presentation. Clin. Exp. Immunol. 1985, 61, 143–151. [Google Scholar]
- Morefield, G.L.; Sokolovska, A.; Jiang, D.; HogenEsch, H.; Robinson, J.; Hem, S.L. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 2005, 23, 1588–1595. [Google Scholar] [CrossRef]
- Ghimire, T.R.; Benson, R.A.; Garside, P.; Brewer, J.M. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol. Lett. 2012, 147, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seubert, A.; Monaci, E.; Pizza, M.; O’Hagan, D.T.; Wack, A. The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells. J. Immunol. 2008, 180, 5402–5412. [Google Scholar] [CrossRef]
- Cioncada, R.; Maddaluno, M.; Vo, H.T.M.; Woodruff, M.; Tavarini, S.; Sammicheli, C.; Tortoli, M.; Pezzicoli, A.; De Gregorio, E.; Carroll, M.C.; et al. Vaccine Adjuvant MF59 Promotes the Intranodal Differentiation of Antigen-Loaded and Activated Monocyte-Derived Dendritic Cells. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef] [Green Version]
- O’Hagan, D.T.; Ott, G.S.; Van Nest, G.; Rappuoli, R.; Del Giudice, G. The history of MF59® adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine Adjuvants: Mode of Action. Front. Immunol. 2013, 4, 214. [Google Scholar] [CrossRef] [Green Version]
- Krieg, A.M. CpG DNA: Trigger of Sepsis, Mediator of Protection, or Both? Scand. J. Infect. Dis. 2003, 35, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M. Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin. Biol. Ther. 2004, 4, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Wilson, H.L.; Lai, K.; Babiuk, L.A.; Mutwiri, G. Activation of Adjuvant Core Response Genes by the Novel Adjuvant PCEP. Mol. Immunol. 2012, 51, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Kool, M.; Willart, M.A.; Hammad, H. Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 2009, 21, 23–29. [Google Scholar] [CrossRef]
- Kool, M.; Soullié, T.; van Nimwegen, M.; Willart, M.A.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008, 205, 869–882. [Google Scholar] [CrossRef] [Green Version]
- Eisenbarth, S.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial Role for the Nalp3 Inflammasome in the Immunostimulatory Properties of Aluminium Adjuvants. Nat. Cell Biol. 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and Innate Immunity: New Perspectives on Host Defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef]
- Poon, C.; Patel, A.A. Organic and inorganic nanoparticle vaccines for prevention of infectious diseases. Nano Express 2020, 1, 012001. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Gill, H.S. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine 2015, 33, 2307–2315. [Google Scholar] [CrossRef] [Green Version]
- Safari, D.; Marradi, M.; Chiodo, F.; Dekker, H.A.T.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold nanoparticles as carriers for a syntheticStreptococcus pneumoniaetype 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.C.D.P.; De Barros, A.L.B.; Belardi, R.M.; De Goes, A.M.; Souza, B.K.D.O.; Soares, D.C.F. Mesoporous silica nanoparticles as a potential vaccine adjuvant against Schistosoma mansoni. J. Drug Deliv. Sci. Technol. 2016, 35, 234–240. [Google Scholar] [CrossRef]
- Guo, H.-C.; Feng, X.-M.; Sun, S.-Q.; Wei, Y.-Q.; Sun, D.-H.; Liu, X.-T.; Liu, Z.-X.; Luo, J.-X.; Yin, H. Immunization of mice by Hollow Mesoporous Silica Nanoparticles as carriers of Porcine Circovirus Type 2 ORF2 Protein. Virol. J. 2012, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, L.M.M.; Zufelato, N.; De Sousa-Júnior, A.A.; Trentini, M.M.; Da Costa, A.C.; Bakuzis, A.; Kipnis, A.; Junqueira-Kipnis, A.P. Specific T Cell Induction Using Iron Oxide Based Nanoparticles as Subunit Vaccine Adjuvant. Hum. Vaccines Immunother. 2018, 14, 2786–2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, M.; Hosseini, S.N.; Khavari-Nejad, R.A.; Najafi, F.; Mahdavi, M. HBs antigen and mannose loading on the surface of iron oxide nanoparticles in order to immuno-targeting: Fabrication, characterization, cellular and humoral immunoassay. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1543–1558. [Google Scholar] [CrossRef]
- Hess, K.; Medintz, I.L.; Jewell, C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 2019, 27, 73–98. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, I.H.; Kang, S.; Kim, D.; Choi, M.; Saw, P.E.; Shin, E.C.; Jon, S. Gold Nanoparticles Displaying Tumor-Associated Self-Antigens as a Potential Vaccine for Cancer Immunotherapy. Adv. Healthc. Mater. 2014, 3, 1194–1199. [Google Scholar] [CrossRef]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles Enabling Co-Delivery of High Amounts of Protein Antigen and Toll-like Receptor 9 Agonist for Enhanced Cancer Vaccine Efficacy. ACS Cent. Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.N.; Kelly, H.G.; Tan, H.; Juno, J.A.; Esterbauer, R.; Davis, T.P.; Truong, N.P.; Wheatley, A.K.; Kent, S.J. Hemagglutinin Functionalized Liposomal Vaccines Enhance Germinal Center and Follicular Helper T Cell Immunity. Adv. Health Mater. 2021, 10, 2002142. [Google Scholar] [CrossRef]
- Huang, W.-C.; Deng, B.; Lin, C.; Carter, K.A.; Geng, J.; Razi, A.; He, X.; Chitgupi, U.; Federizon, J.; Sun, B.; et al. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 2018, 13, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Mansury, D.; Ghazvini, K.; Jamehdar, S.A.; Badiee, A.; Tafaghodi, M.; Nikpoor, A.R.; Amini, Y.; Jaafari, M.R. Enhancement of the effect of BCG vaccine against tuberculosis using DDA/TDB liposomes containing a fusion protein of HspX, PPE44, and EsxV. Artif. Cells Nanomed. Biotechnol. 2019, 47, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Chatzikleanthous, D.; Schmidt, S.T.; Buffi, G.; Paciello, I.; Cunliffe, R.; Carboni, F.; Romano, M.R.; O’Hagan, D.T.; D’Oro, U.; Woods, S.; et al. Design of a Novel Vaccine Nanotechnology-Based Delivery System Comprising CpGODN-Protein Conjugate Anchored to Liposomes. J. Control. Release 2020, 323, 125–137. [Google Scholar] [CrossRef]
- Lanza, J.S.; Vucen, S.; Flynn, O.; Donadei, A.; Cojean, S.; Loiseau, P.M.; Fernandes, A.P.S.; Frézard, F.; Moore, A.C. A TLR9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization. Int. J. Pharm. 2020, 586, 119390. [Google Scholar] [CrossRef] [PubMed]
- Sayour, E.J.; Mendez-Gomez, H.R.; Mitchell, D.A. Cancer Vaccine Immunotherapy with RNA-Loaded Liposomes. Int. J. Mol. Sci. 2018, 19, 2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuts, J.; Varypataki, E.M.; Van Der Maaden, K.; Romeijn, S.; Drijfhout, J.W.; Van Scheltinga, A.T.; Ossendorp, F.; Jiskoot, W. Cationic Liposomes: A Flexible Vaccine Delivery System for Physicochemically Diverse Antigenic Peptides. Pharm. Res. 2018, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.A.; Martina, B.; Prakash, J. Nanomedicine strategies to target coronavirus. Nano Today 2020, 35, 100961. [Google Scholar] [CrossRef]
- Feliciello, I.; Procino, A. The Pulmonary-Proteoliposome as a New Therapeutic Approach for Coronaviruses. Hum. Vaccines Immunother. 2020, 16, 2373. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Tretyakova, I. Influenza Virus Like Particles (VLPs): Opportunities for H7N9 Vaccine Development. Viruses 2020, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Kazaks, A.; Lu, I.-N.; Farinelle, S.; Ramirez, A.; Crescente, V.; Blaha, B.; Ogonah, O.; Mukhopadhyay, T.; De Obanos, M.P.; Krimer, A.; et al. Production and purification of chimeric HBc virus-like particles carrying influenza virus LAH domain as vaccine candidates. BMC Biotechnol. 2017, 17, 79. [Google Scholar] [CrossRef] [Green Version]
- Quan, F.-S.; Basak, S.; Chu, K.-B.; Kim, S.S.; Kang, S.-M. Progress in the development of virus-like particle vaccines against respiratory viruses. Expert Rev. Vaccines 2020, 19, 11–24. [Google Scholar] [CrossRef]
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef]
- Garg, H.; Mehmetoglu-Gurbuz, T.; Joshi, A. Virus Like Particles (VLP) as multivalent vaccine candidate against Chikungunya, Japanese Encephalitis, Yellow Fever and Zika Virus. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.A.; Wetzel, D.; Reiling, L.; Miura, K.; Drew, D.R.; Gilson, P.R.; Anderson, D.A.; Richards, J.S.; Long, C.A.; Suckow, M.; et al. Malaria Vaccine Candidates Displayed on Novel Virus-like Particles Are Immunogenic and Induce Transmission-Blocking Activity. PLoS ONE 2019, 14, e0221733. [Google Scholar] [CrossRef] [Green Version]
- Caldeira, J.C.; Perrine, M.; Pericle, F.; Cavallo, F. Virus-Like Particles as an Immunogenic Platform for Cancer Vaccines. Viruses 2020, 12, 488. [Google Scholar] [CrossRef]
- Swann, H.; Sharma, A.; Preece, B.; Peterson, A.; Eldredge, C.; Belnap, D.M.; Vershinin, M.; Saffarian, S. Minimal System for Assembly of SARS-CoV-2 Virus like Particles. Sci. Rep. 2020, 10, 21877. [Google Scholar] [CrossRef]
- Xu, R.; Shi, M.; Li, J.; Song, P.; Li, N. Construction of SARS-CoV-2 Virus-Like Particles by Mammalian Expression System. Front. Bioeng. Biotechnol. 2020, 8, 1–6. [Google Scholar] [CrossRef]
- Fougeroux, C.; Goksøyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Søgaard, M.; Aves, K.-L.; Horsted, E.W.; et al. Capsid-like Particles Decorated with the SARS-CoV-2 Receptor-Binding Domain Elicit Strong Virus Neutralization Activity. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Chan, S.K.; Du, P.; Ignacio, C.; Mehta, S.; Newton, I.G.; Steinmetz, N.F. Biomimetic Virus-Like Particles as Severe Acute Respiratory Syndrome Coronavirus 2 Diagnostic Tools. ACS Nano 2020. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Danafar, H.; Rahimi, H.; Mozafari, F.; Salehiabar, M.; Rahmati, M.A.; Rahamooz-Haghighi, S.; Mousazadeh, N.; Mohammadi, A.; Ertas, Y.N.; et al. Nanotechnology against the novel coronavirus (severe acute respiratory syndrome coronavirus 2): Diagnosis, treatment, therapy and future perspectives. Nanomedicine 2021, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-Based Biocompatible Nanoparticles for next-Generation Tolerogenic Vaccines against Autoimmune Disease. Int. J. Mol. Sci. 2019, 20, 204. [Google Scholar] [CrossRef] [Green Version]
- Gu, P.; Wusiman, A.; Zhang, Y.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Rational Design of PLGA Nanoparticle Vaccine Delivery Systems To Improve Immune Responses. Mol. Pharm. 2019, 16, 5000–5012. [Google Scholar] [CrossRef]
- Shen, C.; Li, J.; Zhang, Y.; Li, Y.; Shen, G.; Zhu, J.; Tao, J. Polyethylenimine-based micro/nanoparticles as vaccine adjuvants. Int. J. Nanomed. 2017, 12, 5443–5460. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Griffith, T.S.; Panyam, J. Poly(d,l-lactide-co-glycolide) Nanoparticles as Delivery Platforms for TLR7/8 Agonist-Based Cancer Vaccine. J. Pharmacol. Exp. Ther. 2019, 370, 715–724. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, B. Structure-aided ACEI-capped remdesivir-loaded novel PLGA nanoparticles: Toward a computational simulation design for anti-SARS-CoV-2 therapy. Phys. Chem. Chem. Phys. 2020, 22, 28434–28439. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Shibu, M.A.; Toth, I.; Skwarczynskia, M.; Skwarczynski, M.; Skwarczyski, M. Cell-penetrating Peptides: Efficient Vectors for Vaccine Delivery. Curr. Drug Deliv. 2019, 16, 430–443. [Google Scholar] [CrossRef]
- Lim, S.; Koo, J.-H.; Choi, J.-M. Use of cell-penetrating peptides in dendritic cell-based vaccination. Immune Netw. 2016, 16, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Yin, R.; Tian, Y.; Huang, Z.; Shi, J.; Fu, X.; Wang, L.; Wu, Y.; Hao, F.; Ni, B. A novel self-assembled nanoparticle vaccine with HIV-1 Tat49-57/HPV16 E749-57 fusion peptide and GM-CSF DNA elicits potent and prolonged CD8+ T cell-dependent anti-tumor immunity in mice. Vaccine 2012, 30, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Xie, Z.; Zhang, Z.; Gong, T.; Sun, X. Engineering intravaginal vaccines to overcome mucosal and epithelial barriers. Biomaterials 2017, 128, 8–18. [Google Scholar] [CrossRef]
- Chen, X.; Lai, J.; Pan, Q.; Tang, Z.; Yu, Y.; Zang, G. The Delivery of HBcAg via Tat-PTD Enhances Specific Immune Response and Inhibits Hepatitis B Virus Replication in Transgenic Mice. Vaccine 2010, 28, 3913–3919. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Jing, W.; Yingru, X.; Wenyang, W.; Ru, C.; Shengfa, N.; Congjing, X.; Jingjing, D.; Wan, W.; Jiang, H.; et al. Enhanced Anti-Tuberculosis Immunity by a TAT-Ag85B Protein Vaccine in a Murine Tuberculosis Model. Pathog. Glob. Health 2015, 109, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.; Bolhassani, A.; Shojaosadati, S.A.; Aghasadeghi, M.R. MPG-based nanoparticle: An efficient delivery system for enhancing the potency of DNA vaccine expressing HPV16E7. Vaccine 2015, 33, 3164–3170. [Google Scholar] [CrossRef]
- Rostami, B.; Irani, S.; Bolhassani, A.; Cohan, R.A. Gene and protein delivery using four cell penetrating peptides for HIV-1 vaccine development. IUBMB Life 2019, 71, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, S.; Bolhassani, A.; Sadat, S.M.; Irani, S. Design and in Vitro Delivery of HIV-1 Multi-Epitope DNA and Peptide Constructs Using Novel Cell-Penetrating Peptides. Biotechnol. Lett. 2019, 41, 1283–1298. [Google Scholar] [CrossRef]
- Mehrlatifan, S.; Mirnurollahi, S.M.; Motevalli, F.; Rahimi, P.; Soleymani, S.; Bolhassani, A. The structural HCV genes delivered by MPG cell penetrating peptide are directed to enhance immune responses in mice model. Drug Deliv. 2015, 23, 2852–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, J.; Liu, D.; Han, Y.; Xu, H.; Liu, L.; Leng, X.; Kong, D. A cell-penetrating peptide-assisted nanovaccine promotes antigen cross-presentation and anti-tumor immune response. Biomater. Sci. 2019, 7, 5516–5527. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Y.; Xue, W.; Liu, Z. Cell Penetrating Peptide-Based Redox-Sensitive Vaccine Delivery System for Subcutaneous Vaccination. Mol. Pharm. 2018, 15, 975–984. [Google Scholar] [CrossRef]
- Schutze-Redelmeier, M.-P.M.; Kong, S.; Bally, M.B.; Dutz, J.P. Antennapedia transduction sequence promotes anti tumour immunity to epicutaneously administered CTL epitopes. Vaccine 2004, 22, 1985–1991. [Google Scholar] [CrossRef]
- Jarzebska, N.T.; Lauchli, S.; Iselin, C.; French, L.E.; Johansen, P.; Guenova, E.; Kündig, T.M.; Pascolo, S. Functional differences between protamine preparations for the transfection of mRNA. Drug Deliv. 2020, 27, 1231–1235. [Google Scholar] [CrossRef]
- Tusup, M.; French, L.E.; De Matos, M.; Gatfield, D.; Kundig, T.; Pascolo, S. Design of in vitro Transcribed mRNA Vectors for Research and Therapy. Chim. Int. J. Chem. 2019, 73, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Pascolo, S.; Scheel, B.; Derhovanessian, E.; Pflugfelder, A.; Eigentler, T.K.; Pawelec, G.; Hoerr, I.; Rammensee, H.-G.; Garbe, C. Direct Injection of Protamine-protected mRNA: Results of a Phase 1/2 Vaccination Trial in Metastatic Melanoma Patients. J. Immunother. 2009, 32, 498–507. [Google Scholar] [CrossRef]
- Papachristofilou, A.; Hipp, M.M.; Klinkhardt, U.; Früh, M.; Sebastian, M.; Weiss, C.; Pless, M.; Cathomas, R.; Hilbe, W.; Pall, G.; et al. Phase Ib Evaluation of a Self-Adjuvanted Protamine Formulated MRNA-Based Active Cancer Immunotherapy, BI1361849 (CV9202), Combined with Local Radiation Treatment in Patients with Stage IV Non-Small Cell Lung Cancer. J. Immunother. Cancer 2019, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Schnee, M.; Vogel, A.B.; Voss, D.; Petsch, B.; Baumhof, P.; Kramps, T.; Stitz, L. An MRNA Vaccine Encoding Rabies Virus Glycoprotein Induces Protection against Lethal Infection in Mice and Correlates of Protection in Adult and Newborn Pigs. PLoS Negl. Trop. Dis. 2016, 10, 1–20. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and Immunogenicity of a MRNA Rabies Vaccine in Healthy Adults: An Open-Label, Non-Randomised, Prospective, First-in-Human Phase 1 Clinical Trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. MRNA Based SARS-CoV-2 Vaccine Candidate CVnCoV Induces High Levels of Virus Neutralizing Antibodies and Mediates Protection in Rodents. BioRxiv 2020. [Google Scholar] [CrossRef]








| Application Field | Protamine and NPs Applied | References |
|---|---|---|
| Diabetes therapy | Protamine is applied in insulin preparations to form protamine-zinc-insulin complexes as well as Protamine Hagedorn insulin (NPH) in order to prolong the insulin effect. | [10,11] |
| Heparin antagonist | Protamine free base, protamine chloride and Protamine sulfate are applied as antidote against the anticoagulation effect of negatively charged heparin for example in cardiac surgery. | [12,13,14,15] |
| Nanopharmaceuticals | Protamines are noninvasive cell penetrating peptides, showing the ability to target drugs to specific molecules within the cells and form nanoparticles by self-assembling with negatively charged macromolecules. All kinds of (derivatized) protamines (free base, chloride, sulfate, low molecular weight) are forming nanoparticles. Modifications with human serum albumin, polyethylene glycol, citric acid, secretoneurin or packing oligonucleotides (ODN) in solid lipid nanoparticles or liposomes were performed. | [25,27,29,51,52,53] |
| Vaccines | Protamine, used as a carrier for antigenic RNA molecules, in the form of nanoparticles or nanocapsules, can be used as a vaccine and adjuvant. The fields of application include infective diseases, as well as cancer. | [41,43,49,54,55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruseska, I.; Fresacher, K.; Petschacher, C.; Zimmer, A. Use of Protamine in Nanopharmaceuticals—A Review. Nanomaterials 2021, 11, 1508. https://doi.org/10.3390/nano11061508
Ruseska I, Fresacher K, Petschacher C, Zimmer A. Use of Protamine in Nanopharmaceuticals—A Review. Nanomaterials. 2021; 11(6):1508. https://doi.org/10.3390/nano11061508
Chicago/Turabian StyleRuseska, Ivana, Katja Fresacher, Christina Petschacher, and Andreas Zimmer. 2021. "Use of Protamine in Nanopharmaceuticals—A Review" Nanomaterials 11, no. 6: 1508. https://doi.org/10.3390/nano11061508
APA StyleRuseska, I., Fresacher, K., Petschacher, C., & Zimmer, A. (2021). Use of Protamine in Nanopharmaceuticals—A Review. Nanomaterials, 11(6), 1508. https://doi.org/10.3390/nano11061508