Nanoscale Strontium-Substituted Hydroxyapatite Pastes and Gels for Bone Tissue Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strontium-Substituted Nanoscale Hydroxyapatite (SrHA) Paste Preparation Using Rapid-Mixing Wet Precipitation
2.2. Strontium-Substituted Nanoscale Hydroxyapatite Gel Preparation Using Rapid-Mixing Sol-Gel Method
2.3. Material Characterisation
2.3.1. X-ray Diffraction (XRD)
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. X-ray Fluorescence (XRF)
2.3.4. Fourier Transform Infrared Spectroscopy in Attenuated Total Reflectance Mode (FTIR-ATR)
2.3.5. Radiopacity
2.4. In Vitro Biocompatibility of SrHA Pastes and Gels
2.4.1. Direct Biocompatibility of SrHA Pastes and Gels
2.4.2. Indirect Biocompatibility of SrHA Pastes and Gels
3. Results
3.1. Materials Characterisation
3.1.1. X-ray Diffraction
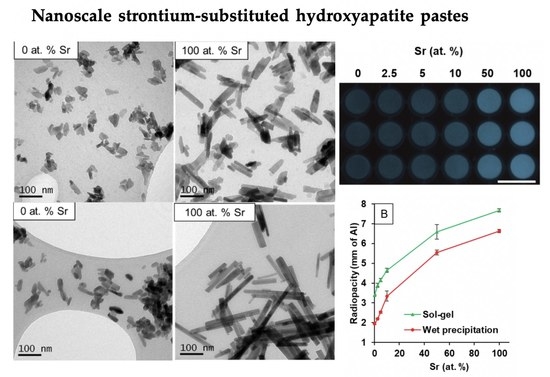
3.1.2. Transmission Electron Microscopy
3.1.3. X-ray Fluorescence
3.1.4. Fourier Transform Infrared Spectroscopy in Attenuated Total Reflectance Mode
3.1.5. Radiopacity
3.2. In Vitro Biocompatibility
3.2.1. Direct Biocompatibility
3.2.2. Indirect Biocompatibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.H.; Hatton, P.V.; Aaron, J.E. The ultrastructure of slam-frozen bone mineral. Histochem. J. 1997, 29, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.X.; Belyaev, O.; Hillmeier, J.; Kock, H.J.; Huber, C.; Meeder, P.J.; Berger, I. First histological observations on the incorporation of a novel nanocrystalline hydroxyapatite paste OSTIM in human cancellous bone. BMC Musculoskelet. Disord. 2006, 7, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruschka, V.; Tangl, S.; Ryabenkova, Y.; Heimel, P.; Barnewitz, D.; Mobus, G.; Keibl, C.; Ferguson, J.; Quadros, P.; Miller, C.; et al. Comparison of nanoparticular hydroxyapatite pastes of different particle content and size in a novel scapula defect model. Sci. Rep. 2017, 7, 43425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Tran, P.A.; Tran, N. Recent advances in research applications of nanophase hydroxyapatite. Chemphyschem 2012, 13, 2495–2506. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Boanini, E.; Gazzano, M.; Giardino, R.; Bigi, A. Interaction of Sr-doped hydroxyapatite nanocrystals with osteoclast and osteoblast-like cells. J. Biomed. Mater. Res. A 2009, 89, 594–600. [Google Scholar] [CrossRef]
- Raucci, M.G.; Giugliano, D.; Alvarez-Perez, M.A.; Ambrosio, L. Effects on growth and osteogenic differentiation of mesenchymal stem cells by the strontium-added sol-gel hydroxyapatite gel materials. J. Mater. Sci. Mater. Med. 2015, 26, 90. [Google Scholar] [CrossRef]
- Buache, E.; Velard, F.; Bauden, E.; Guillaume, C.; Jallot, E.; Nedelec, J.M.; Laurent-Maquin, D.; Laquerriere, P. Effect of strontium-substituted biphasic calcium phosphate on inflammatory mediators production by human monocytes. Acta Biomater. 2012, 8, 3113–3119. [Google Scholar] [CrossRef]
- Dai, J.; Fu, Y.; Chen, D.; Sun, Z. A novel and injectable strontium-containing hydroxyapatite bone cement for bone substitution: A systematic evaluation. Mater. Sci. Eng. C 2021, 124, 112052. [Google Scholar] [CrossRef]
- Yuan, B.; Raucci, M.G.; Fan, Y.; Zhu, X.; Yang, X.; Zhang, X.; Santin, M.; Ambrosio, L. Injectable strontium-doped hydroxyapatite integrated with phosphoserine-tethered poly(epsilon-lysine) dendrons for osteoporotic bone defect repair. J. Mater. Chem. B 2018, 6, 7974–7984. [Google Scholar] [CrossRef]
- Pan, H.B.; Li, Z.Y.; Lam, W.M.; Wong, J.C.; Darvell, B.W.; Luk, K.D.K.; Lu, W.W. Solubility of strontium-substituted apatite by solid titration. Acta Biomater. 2009, 5, 1678–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzavolta, S.; Torricelli, P.; Casolari, S.; Parrilli, A.; Fini, M.; Bigi, A. Strontium-substituted hydroxyapatite-gelatin biomimetic scaffolds modulate bone cell response. Macromol. Biosci. 2018, 18, 1800096. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Park, S.; Miller Ii, A.L.; Terzic, A.; Lu, L. Strontium-substituted hydroxyapatite stimulates osteogenesis on poly(propylene fumarate) nanocomposite scaffolds. J. Biomed. Mater. Res. A 2019, 107, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Boanini, E.; Capuccini, C.; Gazzano, M. Strontium-substituted hydroxyapatite nanocrystals. Inorg. Chim. Acta. 2007, 360, 1009–1016. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Fredholm, Y.; de Rouffignac, A.; Hill, R.G. Structural analysis of a series of strontium-substituted apatites. Acta Biomater. 2008, 4, 1455–1464. [Google Scholar] [CrossRef]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C 2017, 71, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Guo, X.; Cui, W.; Yang, S.; Wang, J.; Qu, Y.; Shao, Z.; Xu, S. Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo. Biomed. Mater. 2018, 13, 015018. [Google Scholar] [CrossRef]
- Bracci, B.; Torricelli, P.; Panzavolta, S.; Boanini, E.; Giardino, R.; Bigi, A. Effect of Mg2+, Sr2+, and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J. Inorg. Biochem. 2009, 103, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Abert, J.; Bergmann, C.; Fischer, H. Wet chemical synthesis of strontium-substituted hydroxyapatite and its influence on the mechanical and biological properties. Ceram. Int. 2014, 40, 9195–9203. [Google Scholar] [CrossRef]
- Gentile, P.; Wilcock, C.J.; Miller, C.A.; Moorehead, R.; Hatton, P.V. Process optimisation to control the physico-chemical characteristics of biomimetic nanoscale hydroxyapatites prepared using wet chemical precipitation. Materials 2015, 8, 2297–2310. [Google Scholar] [CrossRef]
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein. J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef] [Green Version]
- Wilcock, C.J.; Gentile, P.; Hatton, P.V.; Miller, C.A. Rapid mix preparation of bioinspired nanoscale hydroxyapatite for biomedical applications. J. Vis. Exp. 2017, 55343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcock, C.J.; Stafford, G.P.; Miller, C.A.; Ryabenkova, Y.; Fatima, M.; Gentile, P.; Mobus, G.; Hatton, P.V. Preparation and antibacterial properties of silver-doped nanoscale hydroxyapatite pastes for bone repair and augmentation. J. Biomed. Nanotechnol. 2017, 13, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- ISO. BS EN ISO 10993-5:2009. Biological Evaluation of Medical Devices. Tests for in Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Rey, C.; Shimizu, M.; Collins, B.; Glimcher, M.J. Resolution-enhanced fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age: 2. Investigations in the v3 PO4 domain. Calcif. Tissue Int. 1991, 49, 383–388. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Terra, J.; Dourado, E.R.; Eon, J.-G.; Ellis, D.E.; Gonzalez, G.; Rossi, A.M. The structure of strontium-doped hydroxyapatite: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2009, 11, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Mujahid, M.; Amin, S.; Rawat, R.S.; Nusair, A.; Deen, G.R. Effect of surfactant and heat treatment on morphology, surface area and crystallinity in hydroxyapatite nanocrystals. Ceram. Int. 2013, 39, 39–50. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Plūduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Ivanova, T.I.; Frank-Kamenetskaya, O.V.; Kol’tsov, A.B.; Ugolkov, V.L. Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal decomposition. J. Solid State Chem. 2001, 160, 340–349. [Google Scholar] [CrossRef]
- Bouyer, E.; Gitzhofer, F.; Boulos, M.I. Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci. Mater. Med. 2000, 11, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, J.; Wang, Y. Influence of a novel radiopacifier on the properties of an injectable calcium phosphate cement. Acta Biomater. 2007, 3, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Y.; Pan, H.; Lin, K.; Liu, X.; Darvell, B.W.; Lu, W.W.; Chang, J.; Deng, L.; Wang, D.; et al. Effects of strontium in modified biomaterials. Acta Biomater. 2011, 7, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Stipniece, L.; Wilson, S.; Curran, J.M.; Chen, R.; Salma-Ancane, K.; Sharma, P.K.; Meenan, B.J.; Boyd, A.R. Strontium substituted hydroxyapatite promotes direct primary human osteoblast maturation. Ceram. Int. 2021, 47, 3368–3379. [Google Scholar] [CrossRef]
- Crawford, A.; Pinnock, A.; Hruschka, V.; Redl, H.; Hatton, P.V.; Miller, C.A. Biocompatibility of nanohydroxyapatites in 2- and 3-dimensional culture systems. In Proceedings of the PER/International Association for Dental Research (IADR) Congress, Dubrovnik, Croatia, 10–13 September 2014. [Google Scholar]
 hydroxyapatite;
hydroxyapatite;  β-tricalcium phosphate;
β-tricalcium phosphate;  strontium hydroxyapatite;
strontium hydroxyapatite;  β-tristrontium phosphate.
β-tristrontium phosphate.
 hydroxyapatite;
hydroxyapatite;  β-tricalcium phosphate;
β-tricalcium phosphate;  strontium hydroxyapatite;
strontium hydroxyapatite;  β-tristrontium phosphate.
β-tristrontium phosphate.









| Amount of Sr (at.%) | Material Preparation | Characterisation Results | ||||
|---|---|---|---|---|---|---|
| Calcium Hydroxide Amount | Strontium Hydroxide Octahydrate Amount | |||||
| g | mmol | G | mmol | Sr / (Sr + Ca) at.% | (Ca + Sr)/P Molar Ratio | |
| 0 | 3.70 | 50 | 0 | 0 | 0.02 | 1.63 |
| 2.5 | 3.61 | 48.75 | 0.33 | 1.25 | 2.45 | 1.57 |
| 5 | 3.52 | 47.5 | 0.66 | 2.5 | 4.83 | 1.57 |
| 10 | 3.33 | 45 | 1.33 | 5 | 9.55 | 1.56 |
| 50 | 1.85 | 25 | 6.64 | 25 | 49.67 | 1.58 |
| 100 | 0 | 0 | 13.29 | 50 | 99.79 | 1.50 |
| Amount of Sr (at.%) | Material Preparation | Characterisation Results | ||||
|---|---|---|---|---|---|---|
| Calcium Nitrate Tetrahydrate Amount | Strontium Nitrate Amount | |||||
| g | mmol | g | mmol | Sr/(Sr + Ca) at% | (Ca + Sr)/P Molar Ratio | |
| 0 | 11.81 | 50 | 0 | 0 | 0.01 | 1.59 |
| 2.5 | 11.51 | 48.75 | 0.26 | 1.25 | 2.32 | 1.56 |
| 5 | 11.22 | 47.5 | 0.53 | 2.5 | 4.81 | 1.50 |
| 10 | 10.63 | 45 | 1.06 | 5 | 9.01 | 1.54 |
| 50 | 5.90 | 25 | 5.29 | 25 | 45.11 | 1.48 |
| 100 | 0 | 0 | 10.58 | 50 | 99.91 | 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, C.J.; Hatton, P.V.; Gentile, P.; Miller, C.A. Nanoscale Strontium-Substituted Hydroxyapatite Pastes and Gels for Bone Tissue Regeneration. Nanomaterials 2021, 11, 1611. https://doi.org/10.3390/nano11061611
Harrison CJ, Hatton PV, Gentile P, Miller CA. Nanoscale Strontium-Substituted Hydroxyapatite Pastes and Gels for Bone Tissue Regeneration. Nanomaterials. 2021; 11(6):1611. https://doi.org/10.3390/nano11061611
Chicago/Turabian StyleHarrison, Caroline J., Paul V. Hatton, Piergiorgio Gentile, and Cheryl A. Miller. 2021. "Nanoscale Strontium-Substituted Hydroxyapatite Pastes and Gels for Bone Tissue Regeneration" Nanomaterials 11, no. 6: 1611. https://doi.org/10.3390/nano11061611
APA StyleHarrison, C. J., Hatton, P. V., Gentile, P., & Miller, C. A. (2021). Nanoscale Strontium-Substituted Hydroxyapatite Pastes and Gels for Bone Tissue Regeneration. Nanomaterials, 11(6), 1611. https://doi.org/10.3390/nano11061611