Shedding Light on the Chemistry and the Properties of Münchnone Functionalized Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.3. Synthesis of Münchnone Functionalized Graphene
2.4. Synthesis of Thiol Münchnone Functionalized Graphene
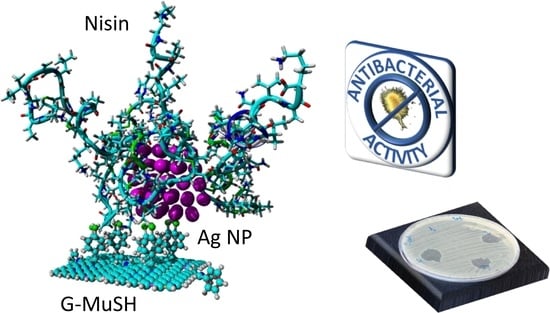
2.5. Synthesis of Nisin-Coated Ag NPs and Graphene Nisin-Coated Ag NP Nanocomposite
2.6. Antimicrobial Activity
2.7. Computational Details
3. Results and Discussion
3.1. Synthesis and Characterization of Münchnone Functionalized Graphene
3.2. Computational Studies
3.3. Synthesis of Graphene Nisin-Coated Ag NPs Nanocomposite
3.4. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grassi, G.; Scala, A.; Piperno, A.; Iannazzo, D.; Lanza, M.; Milone, C.; Pistone, A.; Galvagno, S. A facile and ecofriendly functionalization of multiwalled carbon nanotubes by an old mesoionic compound. Chem. Commun. 2012, 48, 6836–6838. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, Y.J.; Huang, Z.; Huang, X.Y. Pyrrolidine-functionalized fluorine-containing graphene sheets. New J. Chem. 2015, 39, 9586–9590. [Google Scholar] [CrossRef]
- Ren, H.; Cunha, E.; Sun, Q.J.; Li, Z.L.; Kinloch, I.A.; Young, R.J.; Fan, Z.D. Surface functionality analysis by Boehm titration of graphene nanoplatelets functionalized via a solvent-free cycloaddition reaction. Nanoscale Adv. 2019, 1, 1432–1441. [Google Scholar] [CrossRef] [Green Version]
- Plumet, J. 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides under “Non-Conventional” Conditions: Green Solvents, Irradiation, and Continuous Flow. Chempluschem 2020, 85, 2252–2271. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Scala, A.; Fazio, E.; Mineo, P.G.; Rescifina, A.; Piperno, A.; Grassi, G. Repurposing of oxazolone chemistry: Gaining access to functionalized graphene nanosheets in a top-down approach from graphite. Chem. Sci. 2015, 6, 6961–6970. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.; Kumar, N.S.; Chowhan, L.R. Heterogeneous graphene oxide as recyclable catalyst for azomethine ylide mediated 1,3 dipolar cycloaddition reaction in aqueous medium. RSC Adv. 2018, 8, 35587–35593. [Google Scholar] [CrossRef] [Green Version]
- Ferrandiz-Saperas, M.; Ghisolfi, A.; Cazorla-Amoros, D.; Najera, C.; Sansano, J.M. Multilayer graphene functionalized through thermal 1,3-dipolar cycloadditions with imino esters: A versatile platform for supported ligands in catalysis. Chem. Commun. 2019, 55, 7462–7465. [Google Scholar] [CrossRef]
- Neri, G.; Fazio, E.; Mineo, P.G.; Scala, A.; Piperno, A. SERS Sensing Properties of New Graphene/Gold Nanocomposite. Nanomaterials 2019, 9, 1236. [Google Scholar] [CrossRef] [Green Version]
- Foti, C.; Mineo, P.G.; Nicosia, A.; Scala, A.; Neri, G.; Piperno, A. Recent Advances of Graphene-Based Strategies for Arsenic Remediation. Front. Chem. 2020, 8, 608236. [Google Scholar] [CrossRef]
- Stergiou, A.; Canton-Vitoria, R.; Psarrou, M.N.; Economopoulos, S.P.; Tagmatarchis, N. Functionalized graphene and targeted applications-Highlighting the road from chemistry to applications. Prog. Mater. Sci. 2020, 114, 100683. [Google Scholar] [CrossRef]
- Sundramoorthy, A.K.; Gunasekaran, S. Applications of graphene in quality assurance and safety of food. Trac-Trends Anal. Chem. 2014, 60, 36–53. [Google Scholar] [CrossRef]
- Caccamo, D.; Curro, M.; Ientile, R.; Verderio, E.A.M.; Scala, A.; Mazzaglia, A.; Pennisi, R.; Musarra-Pizzo, M.; Zagami, R.; Neri, G.; et al. Intracellular Fate and Impact on Gene Expression of Doxorubicin/Cyclodextrin-Graphene Nanomaterials at Sub-Toxic Concentration. Int. J. Mol. Sci. 2020, 21, 4891. [Google Scholar] [CrossRef]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3-Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef] [Green Version]
- Uceta, H.; Vizuete, M.; Carrillo, J.R.; Barrejon, M.; Fierro, J.L.G.; Prieto, M.P.; Langa, F. Cycloaddition of Nitrile Oxides to Graphene: A Theoretical and Experimental Approach. Chem. A Eur. J. 2019, 25, 14644–14650. [Google Scholar] [CrossRef] [PubMed]
- Barrejon, M.; Gomez-Escalonilla, M.J.; Fierro, J.L.G.; Prieto, P.; Carrillo, J.R.; Rodriguez, A.M.; Abellan, G.; Lopez-Escalante, M.C.; Gabas, M.; Lopez-Navarrete, J.T.; et al. Modulation of the exfoliated graphene work function through cycloaddition of nitrile imines. Phys. Chem. Chem. Phys. 2016, 18, 29582–29590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, A.; Cordaro, M.; Mazzaglia, A.; Risitano, F.; Venuti, A.; Sciortino, M.T.; Grassi, G. Aldol-type compounds from water-soluble indole-3,4-diones: Synthesis, kinetics, and antiviral properties. Mol. Divers. 2013, 17, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Piperno, A.; Scala, A.; Risitano, F.; Grassi, G. Oxazol-5-(4H)-Ones. Part 1. Synthesis and Reactivity as 1,3-dipoles. Curr. Org. Chem. 2014, 18, 2691–2710. [Google Scholar] [CrossRef]
- Barreca, D.; Neri, G.; Scala, A.; Fazio, E.; Gentile, D.; Rescifina, A.; Piperno, A. Covalently immobilized catalase on functionalized graphene: Effect on the activity, immobilization efficiency, and tetramer stability. Biomater. Sci. 2018, 6, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Brancatelli, G.; Bruno, G.; Nicolo, F.; Cordaro, M.; Grassi, G.; Risitano, F.; Scala, A. Crystallographic and theoretical studies of (Z)/(E)-3-phenyl-4-(arylidene) isoxazol-5(4H)-ones. J. Mol. Struct. 2011, 998, 157–166. [Google Scholar] [CrossRef]
- Coros, M.; Varodi, C.; Pogacean, F.; Gal, E.; Pruneanu, S.M. Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate. Sensors 2020, 20, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, A.; Lefevre, X.; Jegou, P.; Deniau, G.; Palacin, S. Spontaneous Grafting of Diazonium Salts: Chemical Mechanism on Metallic Surfaces. Langmuir 2012, 28, 11776–11787. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.M.; Zhang, Y.; Jiang, C.; Qj, M.; Liu, G.Z. Advances on Aryldiazonium Salt Chemistry Based Interfacial Fabrication for Sensing Applications. ACS Appl. Mater. Interfaces 2017, 9, 5031–5049. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Scala, A.; Sortino, G.; Zagami, R.; Zhu, Y.; Sciortino, M.T.; Pennisi, R.; Pizzo, M.M.; Neri, G.; Grassi, G.; et al. Intracellular trafficking and therapeutic outcome of multiwalled carbon nanotubes modified with cyclodextrins and polyethylenimine. Colloids Surf. B. Biointerfaces 2018, 163, 55–63. [Google Scholar] [CrossRef]
- Gentil, S.; Pifferi, C.; Rousselot-Pailley, P.; Tron, T.; Renaudet, O.; Le Goff, A. Clicked Bifunctional Dendrimeric and Cyclopeptidic Addressable Redox Scaffolds for the Functionalization of Carbon Nanotubes with Redox Molecules and Enzymes. Langmuir 2021, 37, 1001–1011. [Google Scholar] [CrossRef]
- Muller-Auffermann, K.; Grijalva, F.; Jacob, F.; Hutzler, M. Nisin and its usage in breweries: A review and discussion. J. Inst. Brew. 2015, 121, 309–319. [Google Scholar] [CrossRef]
- Weinstein, M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institut: Wayne, PA, USA, 2018; p. 112. [Google Scholar]
- Valero, R.; Costa, R.; Moreira, I.D.P.R.; Truhlar, D.G.; Illas, F. Performance of the M06 family of exchange-correlation functionals for predicting magnetic coupling in organic and inorganic molecules. J. Chem. Phys. 2008, 128, 114103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Hehre, W.J. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986; p. xviii. 548p. [Google Scholar]
- Fukui, K. The path of chemical reactions—the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Headgordon, M.; Pople, J.A. A Method for 2-Electron Gaussian Integral and Integral Derivative Evaluation Using Recurrence Relations. J. Chem. Phys. 1988, 89, 5777–5786. [Google Scholar] [CrossRef]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16 Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Cao, Y.; Houk, K.N. Computational assessment of 1,3-dipolar cycloadditions to graphene. J. Mater. Chem. 2011, 21, 1503–1508. [Google Scholar] [CrossRef]
- Bian, S.D.; Scott, A.M.; Cao, Y.; Liang, Y.; Osuna, S.; Houk, K.N.; Braunschweig, A.B. Covalently Patterned Graphene Surfaces by a Force-Accelerated Diels-Alder Reaction. J. Am. Chem. Soc. 2013, 135, 9240–9243. [Google Scholar] [CrossRef] [PubMed]
- Plasser, F.; Pasalic, H.; Gerzabek, M.H.; Libisch, F.; Reiter, R.; Burgdorfer, J.; Muller, T.; Shepard, R.; Lischka, H. The Multiradical Character of One- and Two-Dimensional Graphene Nanoribbons. Angew. Chem. Int. Ed. 2013, 52, 2581–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendikov, M.; Duong, H.M.; Starkey, K.; Houk, K.N.; Carter, E.A.; Wudl, F. Oligoacenes: Theoretical prediction of open-shell singlet diradical ground states. J. Am. Chem. Soc. 2004, 126, 7416–7417. [Google Scholar] [CrossRef]
- Lazar, P.; Karlicky, F.; Jurecka, P.; Kocman, M.; Otyepkova, E.; Safarova, K.; Otyepka, M. Adsorption of Small Organic Molecules on Graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef]
- Lopchuk, J.M.; Hughes, R.P.; Gribble, G.W. What Controls Regiochemistry in 1,3-Dipolar Cycloadditions of Munchnones with Nitrostyrenes? Org. Lett. 2013, 15, 5218–5221. [Google Scholar] [CrossRef]
- Martin-Rodriguez, M.; Castello, L.M.; Najera, C.; Sansano, J.M.; Larranaga, O.; De Cozar, A.; Cossio, F.P. Synthetic scope and DFT analysis of the chiral binap-gold(I) complex-catalyzed 1,3-dipolar cycloaddition of azlactones with alkenes. Beilstein J. Org. Chem. 2013, 9, 2422–2433. [Google Scholar] [CrossRef] [Green Version]
- Denis, P.A. Organic Chemistry of Graphene: The Diels-Alder Reaction. Chem. A Eur. J. 2013, 19, 15719–15725. [Google Scholar] [CrossRef]
- Neri, G.; Scala, A.; Barreca, F.; Fazio, E.; Mineo, P.G.; Mazzaglia, A.; Grassi, G.; Piperno, A. Engineering of carbon based nanomaterials by ring-opening reactions of a reactive azlactone graphene platform. Chem. Commun. 2015, 51, 4846–4849. [Google Scholar] [CrossRef]
- Piperno, A.; Mazzaglia, A.; Scala, A.; Pennisi, R.; Zagami, R.; Neri, G.; Torcasio, S.M.; Rosmini, C.; Mineo, P.G.; Potara, M.; et al. Casting Light on Intracellular Tracking of a New Functional Graphene-Based MicroRNA Delivery System by FLIM and Raman Imaging. ACS Appl. Mater. Interfaces 2019, 11, 46101–46111. [Google Scholar] [CrossRef]
- Abts, A.; Mavaro, A.; Stindt, J.; Bakkes, P.J.; Metzger, S.; Driessen, A.J.M.; Smits, S.H.J.; Schmitt, L. Easy and Rapid Purification of Highly Active Nisin. Int. J. Pept. 2011, 2011, 175145. [Google Scholar] [CrossRef]
- Scala, A.; Piperno, A.; Hada, A.; Astilean, S.; Vulpoi, A.; Ginestra, G.; Marino, A.; Nostro, A.; Zammuto, V.; Gugliandolo, C. Marine Bacterial Exopolymers-Mediated Green Synthesis of Noble Metal Nanoparticles with Antimicrobial Properties. Polymers 2019, 11, 1157. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.Y.; Na, H.Y.; Zhong, R.B.; Yuan, M.; Guo, J.; Zhao, L.J.; Wang, Y.; Wang, L.P.; Zhang, F. One step synthesis of antimicrobial peptide protected silver nanoparticles: The core-shell mutual enhancement of antibacterial activity. Colloids Surf. B. Biointerfaces 2020, 186, 110704. [Google Scholar] [CrossRef]
- Neri, G.; Cordaro, A.; Scala, A.; Cordaro, M.; Mazzaglia, A.; Piperno, A. PEGylated bis-adamantane carboxamide as guest bridge for graphene poly-cyclodextrin gold nanoassemblies. J. Mol. Struct. 2021, 1240, 130519. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. Graphene Oxide-Silver Nanoparticle Nanohybrids: Synthesis, Characterization, and Antimicrobial Properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Nicosia, A.; Vento, F.; Pellegrino, A.L.; Ranc, V.; Piperno, A.; Mazzaglia, A.; Mineo, P. Polymer-Based Graphene Derivatives and Microwave-Assisted Silver Nanoparticles Decoration as a Potential Antibacterial Agent. Nanomaterials 2020, 10, 2269. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Corsaro, C.; Fazio, E. Plasmon-Enhanced Controlled Drug Release from Ag-PMA Capsules. Molecules 2020, 25, 2267. [Google Scholar] [CrossRef] [PubMed]
- Mosselhy, D.A.; El-Aziz, M.A.; Hanna, M.; Ahmed, M.A.; Husien, M.M.; Feng, Q.L. Comparative synthesis and antimicrobial action of silver nanoparticles and silver nitrate. J. Nanopart. Res. 2015, 17, 473. [Google Scholar] [CrossRef]












| G-Mu1 | G-Mu2 | GF | |
|---|---|---|---|
| C 1s (%) | 93.1 | 92.7 | 88.4 |
| O 1s (%) | 5.6 | 6.4 | 11.6 |
| N 1s (%) | 1.3 | 0.9 | 0.0 |
| Structure | Direct ΔG a | Inverse ΔG b |
|---|---|---|
| TS-a1 | 15.6 | 31.0 |
| TS-a2 | 19.1 | 42.7 |
| TS-b | 42.8 | 8.4 |
| TS-c1 | 12.6 | 6.3 |
| TS-c2 | 12.7 | 18.1 |
| TS-c3 | 19.8 | 54.3 |
| I-a1 | −15.4 | — |
| I-b | 34.4 | — |
| I-c1 | 6.3 | — |
| I-c2 | 0.9 | — |
| G-Mu-a | −39.0 | — |
| G-Mu-c | −33.6 | — |
| Commercial Nisin | Nisin@Ag | G-MuSH/Nisin@Ag | ||
|---|---|---|---|---|
| S. aureus | MIC a | 281.25 ≡ [Nisin] = 7.03 | 66.67 ≡ [Nisin] = 1.39 and [Ag] = 5.62 | 178.75 ≡ [Nisin] = 2.79 and [Ag] = 11.25 |
| MBC b | 2250 ≡ [Nisin] = 56.25 | 535 ≡ [Nisin] = 11.15 and [Ag] = 45 | NA c | |
| E. coli | MIC a | NAc | 66.67 ≡ [Nisin] = 1.39 and [Ag] = 5.62 | 178.75 ≡ [Nisin] = 2.79 and [Ag] = 11.25 |
| MBC b | NAc | 133 ≡ [Nisin] = 2.79 and [Ag] = 11.25 | 178.75 ≡ [Nisin] = 2.79 and [Ag] = 11.25 | |
| P. aeruginosa | MIC a | NAc | 133 ≡ [Nisin] = 2.79 and [Ag] = 11.25 | 357.5 ≡ [Nisin] = 5.57 and [Ag] = 22.5 |
| MBC b | NAc | 133 ≡ [Nisin] = 2.79 and [Ag] = 11.25 | 357.5 ≡ [Nisin] = 5.57 and [Ag] = 22.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, G.; Fazio, E.; Nostro, A.; Mineo, P.G.; Scala, A.; Rescifina, A.; Piperno, A. Shedding Light on the Chemistry and the Properties of Münchnone Functionalized Graphene. Nanomaterials 2021, 11, 1629. https://doi.org/10.3390/nano11071629
Neri G, Fazio E, Nostro A, Mineo PG, Scala A, Rescifina A, Piperno A. Shedding Light on the Chemistry and the Properties of Münchnone Functionalized Graphene. Nanomaterials. 2021; 11(7):1629. https://doi.org/10.3390/nano11071629
Chicago/Turabian StyleNeri, Giulia, Enza Fazio, Antonia Nostro, Placido Giuseppe Mineo, Angela Scala, Antonio Rescifina, and Anna Piperno. 2021. "Shedding Light on the Chemistry and the Properties of Münchnone Functionalized Graphene" Nanomaterials 11, no. 7: 1629. https://doi.org/10.3390/nano11071629
APA StyleNeri, G., Fazio, E., Nostro, A., Mineo, P. G., Scala, A., Rescifina, A., & Piperno, A. (2021). Shedding Light on the Chemistry and the Properties of Münchnone Functionalized Graphene. Nanomaterials, 11(7), 1629. https://doi.org/10.3390/nano11071629