Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of QD2
2.3. Conjugation of Anti-PSA Abs onto QD2 (QD2-PSA Ab)
2.4. Characterization of QD2 and QD2-PSA Ab
2.5. Preparation of Test Strips
2.6. Preparation of Clinical Samples of Human Serum
2.7. Analysis of PSA and Human Serum Using the Prepared Test Strips
2.8. Photoluminescence Intensity Measurement of the Prepared Test Strips
3. Results and Discussion
3.1. Characterization of QD2 and QD2-PSA Ab
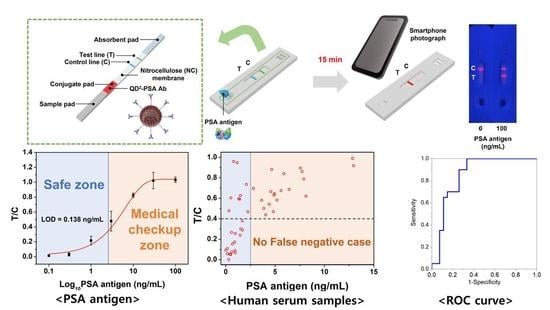
3.2. Detection of PSA Using the QD2-Based LFIA Test Strip
3.3. Detection of PSA in Human Serum Using the QD2-Based LFIA Test Strip
3.4. Selectivity Test of the Developed LFIA System
3.5. Stability Test of the Developed LFIA System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Etzioni, R.; Tsodikov, A.; Mariotto, A.; Szabo, A.; Falcon, S.; Wegelin, J.; Karnofski, K.; Gulati, R.; Penson, D.F.; Feuer, E. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008, 19, 175–181. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Cozzi, P.; Walsh, B.; Willcox, M.; Kearsley, J.; Russell, P.; Li, Y. Innovative biomarkers for prostate cancer early diagnosis and progression. Crit. Rev. Oncol. Hematol. 2010, 73, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Beyond PSA: The next generation of prostate cancer biomarkers. Sci. Transl. Med. 2012, 4, 127rv3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, M.J. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N. Engl. J. Med. 2001, 344, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Gore, J.L.; Etzioni, R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: Model estimates of potential benefits and harms. Ann. Intern. Med. 2013, 158, 145–153. [Google Scholar] [CrossRef] [Green Version]
- De La Rica, R.; Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef]
- McJimpsey, E.L. Molecular form differences between prostate-specific antigen (PSA) standards create quantitative discordances in PSA elisa measurements. Sci. Rep. 2016, 6, 22050. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Huynh, K.-H.; Son, B.S.; Kim, H.-M.; Jun, B.-H. Sensitive Colorimetric Detection of Prostate Specific Antigen Using a Peroxidase-Mimicking Anti-PSA Antibody Coated Au Nanoparticle. Biochip J. 2020, 14, 158–168. [Google Scholar] [CrossRef]
- Damborska, D.; Bertok, T.; Dosekova, E.; Holazova, A.; Lorencova, L.; Kasak, P.; Tkac, J. Nanomaterial-based biosensors for detection of prostate specific antigen. Mikrochim. Acta 2017, 184, 3049–3067. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Sink, T.; Lochmann, R.; Fecteau, K. Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiol. Biochem. 2008, 34, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Di Nardo, F.; Roda, A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015, 64, 63–68. [Google Scholar] [CrossRef]
- Liu, C.; Jia, Q.; Yang, C.; Qiao, R.; Jing, L.; Wang, L.; Xu, C.; Gao, M. Lateral flow immunochromatographic assay for sensitive pesticide detection by using Fe3O4 nanoparticle aggregates as color reagents. Anal. Chem. 2011, 83, 6778–6784. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Sabour, A.F.; Son, J.H.; Lee, S.H.; Lee, L.P. Toward Integrated Molecular Diagnostic System (i MDx): Principles and Applications. IEEE. Trans. Biomed. 2014, 61, 1506–1521. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Xu, Y.; Zhao, X.; Li, Q. Lateral flow immunoassay using europium chelate-loaded silica nanoparticles as labels. Clin. Chem. 2009, 55, 179–182. [Google Scholar] [CrossRef]
- Wong, R.; Tse, H. Lateral Flow Immunoassay; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar]
- Parolo, C.; de la Escosura-Muñiz, A.; Merkoçi, A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-M.; Kim, J.; An, J.; Bock, S.; Pham, X.-H.; Huynh, K.-H.; Choi, Y.; Hahm, E.; Song, H.; Kim, J.-W. Au-Ag assembled on silica nanoprobes for visual semiquantitative detection of prostate-specific antigen. J. Nanobiotechnol. 2021, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, O.A.; Guhrenz, C.; Schneider, K.; Beloglazova, N.V.; Goryacheva, I.Y.; De Saeger, S.; Gaponik, N. Silanized luminescent quantum dots for the simultaneous multicolor lateral flow immunoassay of two mycotoxins. ACS Appl. Mater. Interfaces 2020, 12, 24575–24584. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.O.; Covián, L.B.; García, A.C.; Blanco-López, M.C. Silver and gold enhancement methods for lateral flow immunoassays. Talanta 2016, 148, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Chun, P. Colloidal gold and other labels for lateral flow immunoassays. In Lateral Flow Immunoassay; Springer: Berlin, Germany, 2009; pp. 1–19. [Google Scholar]
- Cai, Y.; Kang, K.; Liu, Y.; Wang, Y.; He, X. Development of a lateral flow immunoassay of C-reactive protein detection based on red fluorescent nanoparticles. Anal. Biochem. 2018, 556, 129–135. [Google Scholar] [CrossRef]
- Lee, L.G.; Nordman, E.S.; Johnson, M.D.; Oldham, M.F. A low-cost, high-performance system for fluorescence lateral flow assays. Biosensors 2013, 3, 360–373. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Spano, G.; Speranskaya, E.S.; Goryacheva, I.Y.; Baggiani, C. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim. Acta 2018, 185, 94. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Yang, Q.; Gong, X.; Guo, W.; Dong, C.; Liu, J.; Xuan, L.; Chang, J. Rapid and quantitative detection of prostate specific antigen with a quantum dot nanobeads-based immunochromatography test strip. ACS Appl. Mater. Interfaces 2014, 6, 6406–6414. [Google Scholar] [CrossRef]
- Rong, Z.; Bai, Z.; Li, J.; Tang, H.; Shen, T.; Wang, Q.; Wang, C.; Xiao, R.; Wang, S. Dual-color magnetic-quantum dot nanobeads as versatile fluorescent probes in test strip for simultaneous point-of-care detection of free and complexed prostate-specific antigen. Biosens. Bioelectron. 2019, 145, 111719. [Google Scholar] [CrossRef]
- Bock, S.; An, J.; Kim, H.M.; Kim, J.; Jung, H.S.; Pham, X.H.; Rho, W.Y.; Jun, B.H. A Lateral Flow Immunoassay for Prostate-Specific Antigen Detection Using Silica-Coated CdSe@ ZnS Quantum Dots. Bull. Korean Chem. Soc. 2020, 41, 989–993. [Google Scholar] [CrossRef]
- Park, S.-M.; Won, D.D.; Lee, B.J.; Escobedo, D.; Esteva, A.; Aalipour, A.; Ge, T.J.; Kim, J.H.; Suh, S.; Choi, E.H. A mountable toilet system for personalized health monitoring via the analysis of excreta. Nat. Biomed. Eng. 2020, 4, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Ge, T.J.; Won, D.D.; Lee, J.K.; Liao, J.C. Digital biomarkers in human excreta. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 521–522. [Google Scholar] [CrossRef]
- Jun, B.H.; Hwang, D.W.; Jung, H.S.; Jang, J.; Kim, H.; Kang, H.; Kang, T.; Kyeong, S.; Lee, H.; Jeong, D.H. Ultrasensitive, Biocompatible, Quantum-Dot-Embedded Silica Nanoparticles for Bioimaging. Adv. Funct. Mater. 2012, 22, 1843–1849. [Google Scholar] [CrossRef]
- Kim, H.-M.; Oh, C.; An, J.; Baek, S.; Bock, S.; Kim, J.; Jung, H.-S.; Song, H.; Kim, J.-W.; Jo, A. Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection. Nanomaterials 2021, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, J.; Bock, S.; An, J.; Choi, Y.-S.; Pham, X.-H.; Cha, M.G.; Seong, B.; Kim, W.; Kim, Y.-H. Silver-Assembled Silica Nanoparticles in Lateral Flow Immunoassay for Visual Inspection of Prostate-Specific Antigen. Sensors 2021, 21, 4099. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.H.; O’Malley, A.J.; Mauri, L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]





| Clinical Sample No. | PSA Concentration (ng/mL) | Clinical Sample No. | PSA Concentration (ng/mL) | Clinical Sample No. | PSA Concentration (ng/mL) |
|---|---|---|---|---|---|
| 1 | 0.001 | 17 | 0.954 | 33 | 4.557 |
| 2 | 0.159 | 18 | 1.146 | 34 | 4.655 |
| 3 | 0.164 | 19 | 1.309 | 35 | 4.815 |
| 4 | 0.300 | 20 | 1.412 | 36 | 4.888 |
| 5 | 0.323 | 21 | 1.455 | 37 | 4.931 |
| 6 | 0.429 | 22 | 1.488 | 38 | 5.182 |
| 7 | 0.479 | 23 | 1.514 | 39 | 5.607 |
| 8 | 0.514 | 24 | 1.677 | 40 | 5.880 |
| 9 | 0.577 | 25 | 1.689 | 41 | 6.418 |
| 10 | 0.619 | 26 | 2.093 | 42 | 7.551 |
| 11 | 0.677 | 27 | 2.233 | 43 | 7.729 |
| 12 | 0.797 | 28 | 2.788 | 44 | 7.900 |
| 13 | 0.897 | 29 | 3.637 | 45 | 8.125 |
| 14 | 0.919 | 30 | 3.847 | 46 | 12.843 |
| 15 | 0.921 | 31 | 4.043 | 47 | 12.950 |
| 16 | 0.945 | 32 | 4.398 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bock, S.; Kim, H.-M.; Kim, J.; An, J.; Choi, Y.-S.; Pham, X.-H.; Jo, A.; Ham, K.-m.; Song, H.; Kim, J.-W.; et al. Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection. Nanomaterials 2022, 12, 33. https://doi.org/10.3390/nano12010033
Bock S, Kim H-M, Kim J, An J, Choi Y-S, Pham X-H, Jo A, Ham K-m, Song H, Kim J-W, et al. Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection. Nanomaterials. 2022; 12(1):33. https://doi.org/10.3390/nano12010033
Chicago/Turabian StyleBock, Sungje, Hyung-Mo Kim, Jaehi Kim, Jaehyun An, Yun-Sik Choi, Xuan-Hung Pham, Ahla Jo, Kyeong-min Ham, Hobeom Song, Jung-Won Kim, and et al. 2022. "Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection" Nanomaterials 12, no. 1: 33. https://doi.org/10.3390/nano12010033
APA StyleBock, S., Kim, H. -M., Kim, J., An, J., Choi, Y. -S., Pham, X. -H., Jo, A., Ham, K. -m., Song, H., Kim, J. -W., Hahm, E., Rho, W. -Y., Lee, S. H., Park, S. -m., Lee, S., Jeong, D. H., Lee, H. -Y., & Jun, B. -H. (2022). Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection. Nanomaterials, 12(1), 33. https://doi.org/10.3390/nano12010033