Spray-Assisted Interfacial Polymerization to Form CuII/I@CMC-PANI Film: An Efficient Dip Catalyst for A3 Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instrumentations
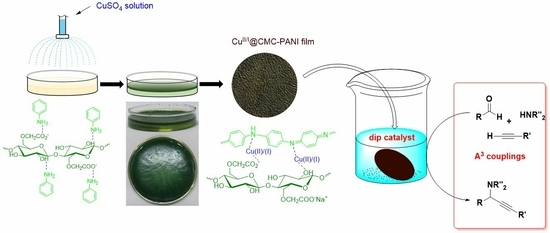
2.2. Preparation of CuII/I@CMC-PANI Film
2.3. General Procedure for A3 Coupling Reactions Catalysed by CuII/I@CMC-PANI Dip Catalyst
3. Results and Discussion
3.1. Synthesis and Characterization of Catalyst
3.2. Application of CuII/I@CMC-PANI Dip Catalyst in Three-Component A3 Coupling Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hariprasad, E.; Radhakrishnan, T.P. A highly efficient and extensively reusable “dip catalyst” based on a silver-nanoparticle-embedded polymer thin film. Chem.-Eur. J. 2010, 16, 14378–14384. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, U.D.; Saha, J.; Radhakrishnan, T.P. ‘Dip Catalysts’ based on polymer-metal nanocomposite thin films: Combining soft-chemical fabrication with efficient application and monitoring. ChemNanoMat 2018, 4, 1191–1201. [Google Scholar] [CrossRef]
- Shaikh, M.N.; Aziz, M.A.; Yamani, Z.H. Facile hydrogenation of cinnamaldehyde to cinnamyl ether by employing a highly re-usable “dip-catalyst” containing Pt nanoparticles on a green support. Catal. Sci. Technol. 2020, 10, 6544–6551. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Anionic polysaccharide stabilized nickel nanoparticles-coated bacterial cellulose as a highly efficient dip-catalyst for pollutants reduction. React. Funct. Polym. 2019, 145, 104395. [Google Scholar] [CrossRef]
- Zheng, G.; Polavarapu, L.; Liz-Marzan, L.M.; Pastoriza-Santos, I.; Perez-Juste, J. Gold nanoparticle-loaded filter paper: A recyclable dip-catalyst for real-time reaction monitoring by surface enhanced Raman scattering. Chem. Commun. 2015, 51, 4572–4575. [Google Scholar] [CrossRef]
- Zheng, G.; Kaefer, K.; Mourdikoudis, S.; Polavarapu, L.; Vaz, B.; Cartmell, S.E.; Bouleghlimat, A.; Buurma, N.J.; Yate, L.; de Lera, A.R.; et al. Palladium nanoparticle-loaded cellulose paper: A highly efficient, robust, and recyclable self-assembled composite catalytic system. J. Phys. Chem. Lett. 2015, 6, 230–238. [Google Scholar] [CrossRef]
- Nishikata, T.; Tsutsumi, H.; Gao, L.; Kojima, K.; Chikama, K.; Nagashima, H. Adhesive catalyst immobilization of palladium nanoparticles on cotton and filter paper: Applications to reusable catalysts for sequential catalytic reactions. Adv. Synth. Catal. 2014, 356, 951–960. [Google Scholar] [CrossRef]
- Madhuri, U.D.; Rao, V.K.; Hariprasad, E.; Radhakrishnan, T.P. In situ fabricated platinum—Poly(vinyl alcohol) nanocomposite thin film: A highly reusable ‘dip catalyst’ for hydrogenation. Mater. Res. Express 2016, 3, 045018. [Google Scholar] [CrossRef]
- Hariprasad, E.; Radhakrishnan, T.P. Palladium nanoparticle-embedded polymer thin film “dip catalyst” for Suzuki–Miyaura reaction. ACS Catal. 2012, 2, 1179–1186. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, G.; Feng, Y.; Li, R.; Hou, P.; Zhang, J.; Wang, J. Facile synthesis of silver nanoparticles on amino-modified cellulose paper and their catalytic properties. J. Mater. Sci. 2017, 53, 1568–1579. [Google Scholar] [CrossRef]
- Feiz, E.; Mahyari, M.; Ghaieni, H.R.; Tavangar, S. Copper on chitosan-modified cellulose filter paper as an efficient dip catalyst for ATRP of MMA. Sci. Rep. 2021, 11, 8257. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Kamal, T.; Khan, S.B.; Asiri, A.M. An efficient and easily retrievable dip catalyst based on silver nanoparticles/chitosan-coated cellulose filter paper. Cellulose 2016, 23, 3577–3588. [Google Scholar] [CrossRef]
- Hemming, D.; Fritzemeier, R.; Westcott, S.A.; Santos, W.L.; Steel, P.G. Copper-boryl mediated organic synthesis. Chem. Soc. Rev. 2018, 47, 7477–7494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmidevi, J.; Naidu, B.R.; Venkateswarlu, K. CuI in biorenewable basic medium: Three novel and low E-factor Suzuki-Miyaura cross-coupling reactions. Mol. Catal. 2022, 522, 112237. [Google Scholar] [CrossRef]
- Venkateswarlu, K. Ashes from organic waste as reagents in synthetic chemistry: A review. Environ. Chem. Lett. 2021, 19, 3887–3950. [Google Scholar] [CrossRef]
- Gan, Z.; Yan, Q.; Li, G.; Li, Q.; Dou, X.; Li, G.Y.; Yang, D. Copper-catalyzed domino synthesis of sulfur-containing heterocycles using carbon disulfide as a building block. Adv. Synth. Catal. 2019, 361, 4558–4567. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Wang, J. Copper-catalyzed direct synthesis of arylated 8-aminoquinolines through chelation assistance. Appl. Organomet. Chem. 2022, 36, e6578. [Google Scholar] [CrossRef]
- Volkova, Y.; Baranin, S.; Zavarzin, I. A3 coupling reaction in the synthesis of heterocyclic compounds. Adv. Synth. Catal. 2020, 363, 40–61. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Ghorbannezhad, F.; Sajadi, S.M. A review on recent advances in the application of nanocatalysts in A3 coupling reactions. Chem. Rec. 2018, 18, 1409–1473. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the A3-coupling. Chem. Soc. Rev. 2012, 41, 3790–3807. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.B.; Wang, S. Interfacial polymerization: From chemistry to functional materials. Angew. Chem. Int. Ed. Engl. 2020, 59, 21840–21856. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.Y.; Krantz, W.B. Formation and characterization of polyamide membranes via interfacial polymerization. J. Membrane Sci. 1994, 93, 175–192. [Google Scholar] [CrossRef]
- Eskandari, E.; Kosari, M.; Farahani, M.H.D.A.; Khiavi, N.D.; Saeedikhani, M.; Katal, R.; Zarinejad, M. A review on polyaniline-based materials applications in heavy metals removal and catalytic processes. Sep. Purif. Technol. 2020, 231, 115901. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Yu, L.; Han, Z.; Ding, Y. Gram-scale preparation of Pd@PANI: A practical catalyst reagent for copper-free and ligand-free sonogashira couplings. Org. Process. Res. Dev. 2016, 20, 2124–2129. [Google Scholar] [CrossRef]
- Yu, L.; Han, Z. Palladium nanoparticles on polyaniline (Pd@PANI): A practical catalyst for Suzuki cross-couplings. Mater. Lett. 2016, 184, 312–314. [Google Scholar] [CrossRef]
- Shi, B.; Zhao, C.; Ji, Y.; Shi, J.; Yang, H. Promotion effect of PANI on Fe-PANI/Zeolite as an active and recyclable Fenton-like catalyst under near-neutral condition. Appl. Surf. Sci. 2020, 508, 145298. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Xie, A.; Chen, S. One-step synthesis of Ag@PANI nanocomposites and their application to detection of mercury. Mater. Chem. Phys. 2013, 140, 487–492. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Su-Yeon, L.; Kil, G.-S.; Park, D.W.; Koo, B.H.; Lee, C.G. Preparation and characterizations of polyaniline (PANI)/ZnO nanocomposites film using solution casting method. Thin Solid Films 2011, 519, 8375–8378. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, N.; Sun, Z.; Li, W.; Sun, Y.; Zhang, H.; Pang, J.; Jiang, Z. Based on confined polymerization: In situ synthesis of PANI/PEEK composite film in one-step. Adv. Sci. 2022, 9, e2103706. [Google Scholar] [CrossRef]
- Heinze, T. New ionic polymers by cellulose functionalization. Macromol. Chem. Phys. 1998, 199, 2341–2364. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, J.; Li, Y. CuSO4 nanoparticles loaded on carboxymethylcellulose/polyaniline composites: A highly efficient catalyst with enhanced catalytic activity in the synthesis of propargylamines, benzofurans, and 1,2,3-triazoles. Appl. Organomet. Chem. 2021, 35, e6349. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Song, Y.; Fan, J.-B.; Wang, S. Recent progress in interfacial polymerization. Mater. Chem. Front. 2017, 1, 1028–1040. [Google Scholar] [CrossRef]
- Megha, R.; Ravikiran, Y.T.; Kotresh, S.; Vijaya Kumari, S.C.; Raj Prakash, H.G.; Thomas, S. Carboxymethyl cellulose: An efficient material in enhancing alternating current conductivity of HCl doped polyaniline. Cellulose 2017, 25, 1147–1158. [Google Scholar] [CrossRef]
- Fathi Achachlouei, B.; Zahedi, Y. Fabrication and characterization of CMC-based nanocomposites reinforced with sodium montmorillonite and TiO2 nanomaterials. Carbohydr. Polym. 2018, 199, 415–425. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, Z.; Li, Y. Carboxymethylcellulose-supported palladium nanoparticles generated in situ from palladium(II) carboxymethylcellulose: An efficient and reusable catalyst for Suzuki–Miyaura and Mizoroki–Heck reactions. Ind. Eng. Chem. Res. 2015, 54, 790–797. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Su, Z. Preparation of PANI–TiO2 nanocomposites and their solid-phase photocatalytic degradation. Polym. Degrad. Stabil. 2006, 91, 2213–2219. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, H.; Liu, G.; Xu, Z. Cu(I)/Cu(II) mixed-valence surface complexes of S-[(2-hydroxyamino)-2-oxoethyl]-N,N-dibutyldithiocarbamate: Hydrophobic mechanism to malachite flotation, J. Colloid Interface Sci. 2018, 512, 701–712. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on artemisia sphaerocephala krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocolloid. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Kai, W. Electrodeposition synthesis of PANI/MnO2/graphene composite materials and its electrochemical performance. Int. J. Electrochem. Sc. 2017, 12, 8306–8314. [Google Scholar] [CrossRef]
- Buron, C.C.; Lakard, B.; Monnin, A.F.; Moutarlier, V.; Lakard, S. Elaboration and characterization of polyaniline films electrodeposited on tin oxides. Synth. Met. 2011, 161, 2162–2169. [Google Scholar] [CrossRef]
- Lee, S.H. The characteristics of Cu2O thin films deposited using RF-magnetron sputtering method with nitrogen-ambient. ETRI J. 2013, 35, 1156–1159. [Google Scholar] [CrossRef]
- Morgan, P.; Partin, D.; Chamberland, B.; O’Keeffe, M. Synthesis of paramelaconite: Cu4O3. J. Solid State Chem. 1996, 121, 33–37. [Google Scholar] [CrossRef]
- Calegari, F.; da Silva, B.C.; Tedim, J.; Ferreira, M.G.S.; Berton, M.A.C.; Marino, C.E.B. Benzotriazole encapsulation in spray-dried carboxymethylcellulose microspheres for active corrosion protection of carbon steel. Prog. Org. Coat. 2020, 138, 105329. [Google Scholar] [CrossRef]
- Kotal, M.; Thakur, A.K.; Bhowmick, A.K. Polyaniline-carbon nanofiber composite by a chemical grafting approach and its supercapacitor application. ACS Appl. Mater. Interfaces 2013, 5, 8374–8386. [Google Scholar] [CrossRef]
- Kidwai, M.; Jahan, A. Nafion® NR50 catalyzed A3-coupling for the synthesis of propargylamines via C-H activation. J. Iran. Chem. Soc. 2011, 8, 462–469. [Google Scholar] [CrossRef]
- Samai, S.; Nandi, G.C.; Singh, M.S. An efficient and facile one-pot synthesis of propargylamines by three-component coupling of aldehydes, amines, and alkynes via C–H activation catalyzed by NiCl2. Tetrahedron Lett. 2010, 51, 5555–5558. [Google Scholar] [CrossRef]
- Zhu, W.; Qian, W.; Zhang, Y. Synthesis of 1, 3-diaryl-3-aminopropynes via the dethiolation of thioamides promoted by the samarium/samarium diiodide mixed reagent. J. Chem. Res. 2005, 2005, 410–412. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, J.; Duan, Z.; Cui, M.; Wu, Y. A simple and economic synthesis of propargylamines by CuI-catalyzed three-component coupling reaction with succinic acid as additive. Aust. J. Chem. 2009, 62, 75–81. [Google Scholar] [CrossRef]
- Namitharan, K.; Pitchumani, K. Nickel-catalyzed solvent-free three-component coupling of aldehyde, alkyne and amine. Eur. J. Org. Chem. 2010, 2010, 411–415. [Google Scholar] [CrossRef]
- Fodor, A.; Kiss, A.; Debreczeni, N.; Hell, Z.; Gresits, I. A simple method for the preparation of propargylamines using molecular sieve modified with copper(II). Org. Biomol. Chem. 2010, 8, 4575–4581. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Hajra, A. Divergent synthesis of allenylsulfonamide and enaminonesulfonamide via In(III)-catalyzed couplings of propargylamine and N-fluorobenzenesulfonimide. J. Org. Chem. 2018, 83, 13157–13165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.-X.; Gao, W.-X.; Ding, J.-C.; Wu, H.-Y. Copper-catalyzed one-pot synthesis of propargylamines via C-H activation in PEG. Appl. Organomet. Chem. 2010, 24, 809–812. [Google Scholar] [CrossRef]
- Sun, R.; Liu, J.; Yang, S.; Chen, M.; Sun, N.; Chen, H.; Xie, X.; You, X.; Li, S.; Liu, Y. Cp2TiCl2-catalyzed cis-hydroalumination of propargylic amines with Red-Al: Stereoselective synthesis of Z-configured allylic amines. Chem. Commun. 2015, 51, 6426–6429. [Google Scholar] [CrossRef]
- Munshi, A.M.; Agarwal, V.; Ho, D.; Raston, C.L.; Saunders, M.; Smith, N.M.; Iyer, K.S. Magnetically directed assembly of nanocrystals for catalytic control of a three-component coupling reaction. Cryst. Growth Des. 2016, 16, 4773–4776. [Google Scholar] [CrossRef]
- Wang, L.; Cai, C. Reusable polymer-anchored amino acid copper complex for the synthesis of propargylamines. J. Chem. Res. 2008, 2008, 538–541. [Google Scholar] [CrossRef]
- Zhou, Y.; He, T.; Wang, Z. Nanoparticles of silver oxide immobilized on different templates: Highly efficient catalysts for three-component coupling of aldehyde-amine-alkyne. Arkivoc 2008, 8, 80–90. [Google Scholar] [CrossRef]
- Han, L.; Li, S.J.; Zhang, X.T.; Tian, S.K. Aromatic aza-claisen rearrangement of arylpropargylammonium salts generated in situ from arynes and tertiary propargylamines. Chem.-Eur. J. 2021, 27, 3091–3097. [Google Scholar] [CrossRef]
- Idzik, K.; Cabaj, J.; Sooducho, J.; Abdel-Fattah, A.A. Classical benzotriazole-mediatedα-aminoalkylations of alkynes: Synthesis and characterization of alk-2-yn-1-amines as amphiphilic materials. Helv. Chim. Acta 2010, 90, 1672–1680. [Google Scholar] [CrossRef]










 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Dose of Catalyst (Mol% of Cu) | Temp. (°C) | Time (h) | Yield b (%) |
| 1 | H2O | 2.7 | 100 | 12 | N.R. c |
| 2 | EtOH | 2.7 | 78 | 12 | 6 |
| 3 | n-BuOH | 2.7 | 110 | 8 | 55 |
| 4 | DMSO | 2.7 | 110 | 8 | 38 |
| 5 | DMF | 2.7 | 110 | 8 | 51 |
| 6 | CH3CN | 2.7 | 80 | 12 | trace d |
| 7 | toluene | 2.7 | 110 | 6 | 97 |
| 8 | toluene | 0 | 110 | 8 | trace d |
| 9 | toluene | 1.8 | 110 | 8 | 93 |
| 10 | toluene | 3.6 | 110 | 8 | 97 |
| 11 | toluene | 2.7 | 90 | 8 | 12 |
| 12 | toluene | 2.7 | 70 | 24 | trace d |
 | |||||
|---|---|---|---|---|---|
| Entry | R | R’ | HNR’’2 | Time (h) | Yield b (%) |
| 1 | 4-Cl-Ph | Ph | morpholine | 6 | 97 (a) |
| 2 | Ph | Ph | morpholine | 8 | 91 (b) |
| 3 | 4-Me-Ph | Ph | morpholine | 8 | 83 (c) |
| 4 | 2-MeO-Ph | Ph | morpholine | 8 | 97 (d) |
| 5 | 2-OH-Ph | Ph | morpholine | 48 | 39 (e) |
| 6 | 3-NO2-Ph | Ph | morpholine | 48 | 86 (f) |
| 7 | Ph | 3-Me-Ph | morpholine | 16 | 87 (g) |
| 8 | Ph | 4-MeO-Ph | morpholine | 32 | 97 (h) |
| 9 | Ph | 4-CF3-Ph | morpholine | 48 | 83 (i) |
| 10 | Ph | 4-Cl-Ph | morpholine | 24 | 88 (j) |
| 11 | Ph | n-hexyl | morpholine | 48 | trace (k) c |
| 12 | Ph | Ph | piperidine | 48 | 84 (l) |
| 13 | 4-Cl-Ph | Ph | piperidine | 48 | 86 (m) |
| 14 | 4-Cl-Ph | Ph | diethylamine | 48 | 58 (n) |
| 15 | Ph | Ph | aniline | 48 | N.P. (o) d |
| 16 | Et | Ph | morpholine | 8 | 54 (p) |
| 17 | i-Pr | Ph | morpholine | 8 | 30 (q) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Xiao, L.; Fan, X.; Lin, D.; Ma, L.; Nie, G.; Li, Y. Spray-Assisted Interfacial Polymerization to Form CuII/I@CMC-PANI Film: An Efficient Dip Catalyst for A3 Reaction. Nanomaterials 2022, 12, 1641. https://doi.org/10.3390/nano12101641
Xu Z, Xiao L, Fan X, Lin D, Ma L, Nie G, Li Y. Spray-Assisted Interfacial Polymerization to Form CuII/I@CMC-PANI Film: An Efficient Dip Catalyst for A3 Reaction. Nanomaterials. 2022; 12(10):1641. https://doi.org/10.3390/nano12101641
Chicago/Turabian StyleXu, Zhian, Liang Xiao, Xuetao Fan, Dongtao Lin, Liting Ma, Guochao Nie, and Yiqun Li. 2022. "Spray-Assisted Interfacial Polymerization to Form CuII/I@CMC-PANI Film: An Efficient Dip Catalyst for A3 Reaction" Nanomaterials 12, no. 10: 1641. https://doi.org/10.3390/nano12101641
APA StyleXu, Z., Xiao, L., Fan, X., Lin, D., Ma, L., Nie, G., & Li, Y. (2022). Spray-Assisted Interfacial Polymerization to Form CuII/I@CMC-PANI Film: An Efficient Dip Catalyst for A3 Reaction. Nanomaterials, 12(10), 1641. https://doi.org/10.3390/nano12101641